You are a scientist looking to determine how Protein A and Protein B interact. You read extensive research on the two proteins and come up with a great experimental plan that requires indirect staining of both targets in your specimen. You scour the literature and find an ideal antibody against Protein A and an ideal antibody against Protein B. The antibodies are both extensively published, knockout validated, and work in your application. But…they are the same isotype and therefore cannot be indirectly probed simultaneously on the sample. What to do?
If you’ve ever found yourself facing a similar problem, then isotype converted, or chimeric, antibodies may be the right solution for you. Read on to learn more.
What is an isotype?
Antibodies are composed of light chains and heavy chains made up of variable regions and constant regions. Variable regions are unique to each antibody and convey specificity to the target while constant regions are identical for antibodies within certain classification groups called isotypes.
Why does isotype matter?
Isotype matters when constraining using the indirect method since a secondary antibody reactive to a certain isotype will bind to all members of that group. If you use two antibodies that are the same isotype then the secondary antibody will bind to both and you will not be able to distinguish between your two targets.
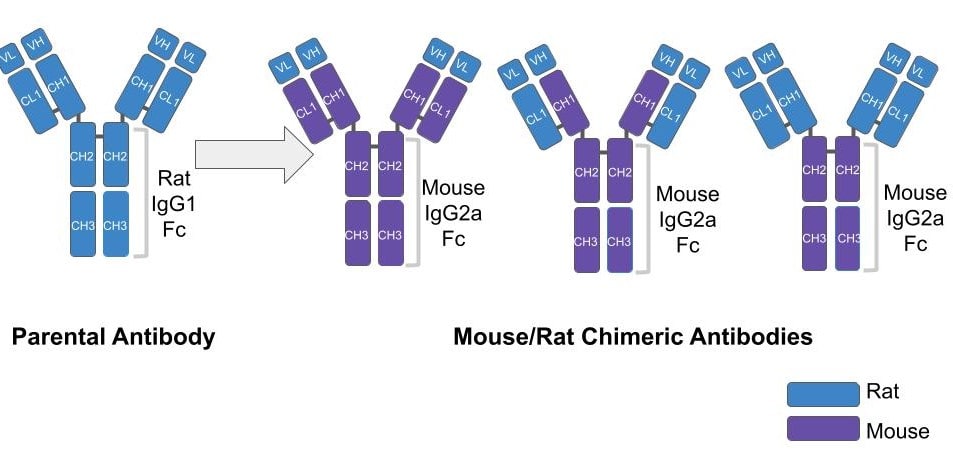 |
| Figure 1: In the isotype conversion process the variable regions of a parental antibody (blue, rat) are cloned into a backbone expressing the constant regions from an alternative isotype (purple, mouse). Depending on the cloning process, all constant regions, just the heavy chain constant regions or solely the Fc region may be changed. |
Chimeric antibodies can help
A chimeric antibody is made by changing an antibody’s natural isotype to that of a different group (Fig. 1). This process, called isotope conversion, gives users the flexibility needed for multiplexing. In the case above, you can’t costain with your desired antibodies because the pair belong to the same isotype and will react with the same secondary. If you were to change one of the antibody’s isotypes, however, you could then use 2 different secondaries for your antibody pair and visualize both targets in parallel (Fig. 2). For example, if both of your antibodies are rat IgG1, then you could convert your anti-Protein B antibody to a mouse IgG2a Fc. The resulting chimeric antibody would have the original variable regions from the parental rat antibody but the Fc of a mouse IgG2a. This would allow you to use a red fluorophore-tagged anti-rat IgG1 secondary to detect Protein A and green fluorophore-tagged anti-mouse IgG2a secondary to detect Protein B and successfully visualize both proteins.
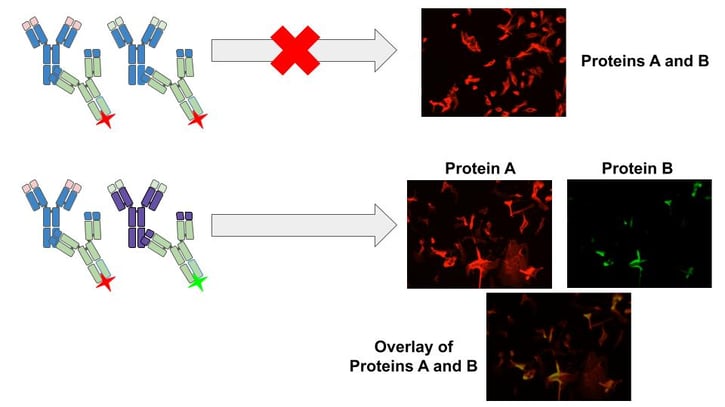 |
|
Figure 2: Two primary antibodies with the same isotype can not be used for multiplexed indirect staining methods since the secondary antibody used will react to both primary antibodies and the targets will be indistinguishable (top panel). If one of the antibodies is converted to a different isotype, then two different secondary antibodies can be used with different fluorescent labels and both targets can be successfully visualized (bottom panel). |
Can any antibody be converted?
Isotype conversion is a unique characteristic of recombinant antibodies. Recombinant antibodies are plasmid-based and are created synthetically or derived from hybridoma antibody sequences that have been cloned into plasmids. Because their sequences are known and encoded on a plasmid, they can be easily manipulated into more useful tools. In the case of isotype conversion, the variable regions of a specific antibody are cloned into a plasmid backbone containing all constant regions, just the heavy chain constant regions or solely the Fc region of a different species and/or isotype (Liddell 2013). In most cases, the resulting chimeric antibody retains the binding specificity to the original target and can be used with secondary antibodies against the new Fc region. In addition to increasing multiplexing capabilities this process is frequently used to reduce immunogenicity and thereby enhance the therapeutic potential of antibody medicines. In this process, antibodies derived from non-human species like mice are “humanized” by replacing as much of the non-antigen binding mouse sequence as possible with human antibody sequences.
Additional considerations
Although isotype conversion is routinely used to increase the utility of recombinant antibody tools, the resulting chimera may have different binding properties (Morelock 1994). While in the majority of cases the differences are subtle, chimeric antibodies should be revalidated before use.
In addition, chimeric antibodies will inevitably retain some sequence from their parental species which can be bound by broadly reactive secondary antibodies to that parental species. While this will not affect single-plexed experiments, it needs to be addressed when multiplexing. It is imperative that you use narrowly selective isotype-specific secondary antibodies when multiplexing with chimeric antibodies. In the case above you should use an anti-rat IgG1 secondary antibody to bind to the anti-Protein A antibody and an anti-mouse IgG2a secondary antibody to bind to the anti-Protein B antibody (Fig. 3)
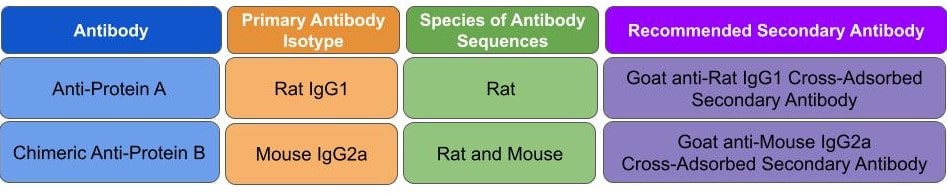 |
| Figure 3: Recommended secondary antibodies for rat IgG1 and mouse 1gG2a primary antibodies used in a multiplexed imaging experiment |
We hope that this blog post has answered all of your questions about the utility of isotype conversions and chimeric antibodies!
References and resources
References
Liddell, E. (2013). Antibodies. In The Immunoassay Handbook (pp. 245–265). Elsevier. https://doi.org/10.1016/b978-0-08-097037-0.00017-8
Morelock, M. M., Rothlein, R., Bright, S. M., Robinson, M. K., Graham, E. T., Sabo, J. P., Owens, R., King, D. J., Norris, S. H., & Scher, D. S. (1994). Isotype choice for chimeric antibodies affects binding properties. In Journal of Biological Chemistry (Vol. 269, Issue 17, pp. 13048–13055). Elsevier BV. https://doi.org/10.1016/s0021-9258(18)99982-5
More resources on the Addgene blog
Antibodies 101: Polyclonal Antibodies
Antibodies 101: Selecting the Right Antibody
Topics: Antibodies







Leave a Comment