When you’re searching for an antibody to use in your next experiment, you’ll probably notice a lot of options to choose from. In this article we’ll cover polyclonal antibodies, one of the many different types of antibodies available (others you’ll encounter include monoclonal and recombinant antibodies). By the end, you’ll have a better understanding of what makes polyclonal antibodies unique and what experiments you should choose them for.
How polyclonal antibodies are produced
Polyclonal antibodies are a heterogeneous mixture of many antibodies that recognize the same protein. In the immune system, antibodies are produced by B cells. Each individual B cell produces antibodies that all recognize the same region, or epitope, of the target protein. These antibodies are also all the same isotype. But together, all the B cell clones in the immune system make different isotypes of antibodies that recognize many different epitopes of the target protein. Whereas production of monoclonal antibodies starts with selecting just one of these B cell clones for further antibody production, polyclonal antibodies include any antibodies produced during the immune response.
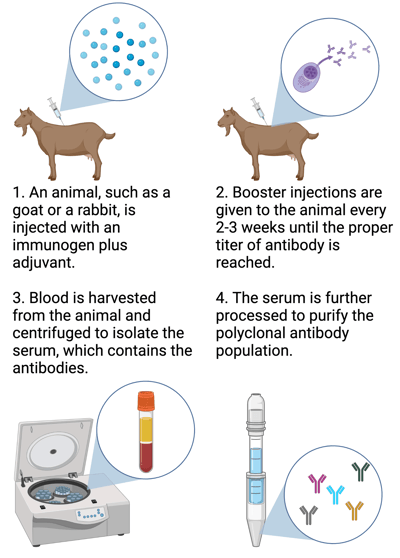 |
| Polyclonal antibodies are generated by injecting an animal with an immunogen, then isolating and purifying the antibodies produced from its serum several weeks later. Created with biorender.com. |
To generate polyclonal antibodies, an animal (such as a rabbit or a goat) is injected with an immunogen. This might be the full-length protein that you want to generate antibodies against, or a unique peptide sequence derived from this protein. At the same time, an adjuvant such as KLH or Freund’s adjuvant is injected, which stimulates the overall immune response. Once the initial antibody response starts to wane about a month later, the animal is given additional booster immunizations every 2-3 weeks to increase the antibody titer. The titer is tested following each immunization until the desired level of antibodies is reached - typically within 2-4 months.
After the animal has the desired level of antibodies in its bloodstream, the antibodies are retrieved first by drawing blood from the immunized animal. The red blood cells and the serum are separated, and the serum - which contains the antibodies - is further processed to isolate the antibodies. Most commonly, antibodies of the IgG isotype are used for research purposes.
Isolating polyclonal antibodies from the serum
There are a few different ways the antibodies can be isolated from the serum.
Protein A/G purification
Protein A or Protein G purification is one common method. These proteins bind the IgG heavy chain. Running the serum over a column of resin crosslinked to protein A /G will bind the IgG antibodies present in the serum, whether specific to the immunogen or not, while any other proteins pass through. However, protein A and protein G each have different affinities for IgG subtypes from different animals so it’s important to choose the reagent that works for your species.
Affinity purification
Only up to 5% of the IgG antibodies produced by an animal are specific for the immunogen. A different type of purification can be performed to isolate antibodies only recognizing your protein of interest: affinity-purified antibodies are isolated from the serum by their ability to bind the target antigen. In this case, the serum is run over a column containing the target protein, and any antibody recognizing the target protein remains bound to the column while the rest of the proteins are eluted. Then, the antibodies are dissociated from the column and from the target protein.
Pre-adsorption
Finally, polyclonal antibodies may be pre-adsorbed to remove antibodies that could cross-react with antibodies of another species. Pre-adsorbed polyclonal antibodies are designed for experiments where antibodies raised in several different species are present. One common example is using pre-adsorbed secondary antibodies (which recognize other antibodies) in experiments that require multiple antibodies that each detect a different antigen.
To pre-adsorb the antibodies, pass them through a column containing immobilized serum protein from other species. Antibodies that don’t cross-react with the serum proteins pass through the column, resulting in a polyclonal antibody population that is enriched for antibodies that recognize your target protein without causing high background due to cross-reactivity.
When to use polyclonal antibodies
One advantage of polyclonal antibodies is that they are inexpensive. Their short production time and the bulk purification steps keep the cost low compared to other antibody formats. This low barrier to entry makes polyclonal antibodies a common reagent in many labs.
Polyclonal antibodies also have a high sensitivity to the target protein due to their ability to recognize many different epitopes of the target protein. While this can increase background staining (see next section), it may be advantageous if you are trying to detect a protein in low abundance or whose conformational state may change. If your protein might become slightly denatured during your experimental protocol, using a polyclonal antibody increases the likelihood that one of the clones will still recognize your protein. For the same reason, they’re also useful in experiments where the availability of some regions of the protein may be masked by crosslinking (ChIP) or fixation (IHC).
Because of their increased sensitivity, polyclonal antibodies are particularly useful for certain research applications. Many secondary antibodies are polyclonal, as their function is to amplify the signal from a primary antibody; the ability of polyclonal antibodies to bind many epitopes of their target protein thereby greatly increasing signal amplification.
When to stay away from polyclonal antibodies
The most prevalent issue with polyclonal antibodies is their batch to batch variability. Once an animal has undergone a terminal bleed to acquire all of its serum, there is no more of that exact polyclonal antibody composition available. If you’re going to need a large quantity of antibody for your experiment, you should either order several vials of the same lot of polyclonal antibody, or select a monoclonal or recombinant antibody instead. Otherwise, you might experience variability in the strength of staining and background noise. If you do use different lots of your polyclonal antibody, you’ll want to use the same positive control across experiments to be aware of signal strength.
In general, polyclonal antibodies produce a higher level of background noise compared to monoclonal or recombinant antibodies. This is because the polyclonal antibody population recognizes many different epitopes, increasing the likelihood of cross-reactivity with other proteins. As with any experiment, when using a polyclonal antibody you’ll want to be sure to choose the appropriate controls to be sure the staining you are seeing is real. It’s important to include a negative control using serum from the same species your polyclonal antibody was generated in or a polyclonal isotype control, to account for nonspecific binding.
Now that you understand the pros and cons of choosing polyclonal antibodies for your experiment and how they compare to other antibody options out there, you’re set up for a successful next experiment!
References and Resources
References
Ascoli CA, Aggeler B (2018) Overlooked benefits of using polyclonal antibodies. BioTechniques 65:127–136. https://doi.org/10.2144/btn-2018-0065
Fishman JB, Berg EA (2019) Antibody Purification and Storage. Cold Spring Harb Protoc 2019:pdb.top099101. https://doi.org/10.1101/pdb.top099101
Additional resources on the Addgene blog
- Read about another common type of antibody for research, monoclonal antibodies
- Learn about the advantages of using recombinant plasmid-based antibodies
Resources on Addgene.org
- Discover Addgene’s antibody plasmid collection
Topics: Antibodies






Leave a Comment