If you’ve been following our antibody blog posts, then you likely already have a good idea of the basics of immunoimaging. A scientist conjugates an antibody with a signaling molecule, the antibody binds to a protein, and then voila! Wherever your protein of interest is, a signal will also be. This is the direct approach to immunoimaging assays. But if the direct approach isn’t giving you enough signal to detect your protein of interest, it may be time to look at the indirect approach - which means it’s time to talk about secondary antibodies.
While a primary antibody binds to the protein of interest, a secondary antibody binds to the primary antibody. In the indirect approach, the signaling molecule is conjugated to the secondary antibody instead of the primary antibody. Since multiple secondary antibodies can bind to a single primary antibody, this approach can greatly increase the sensitivity of an assay by increasing the number of signaling molecules associated, indirectly, with each protein of interest.
|
|
|
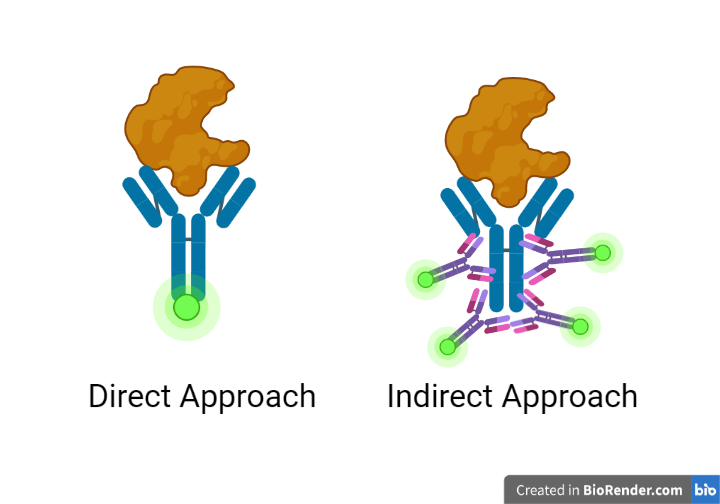
Figure 1: The direct approach (left) versus the indirect approach (right). Primary antibodies are represented in blue; secondary antibodies are represented in purple; signaling molecules are represented in light green; and proteins are represented in orange. Created with BioRender.com.
|
How do secondary antibodies work?
Secondary antibodies are antibodies generated against antibody isotypes from a specific species, such as the rabbit IgG class of antibodies. They do so by targeting the Fc region of an antibody, the so-called ‘constant’ region which is, quite usefully, both conserved across and unique to a species. This Fc region (the stem of an antibody’s Y) is large enough to allow multiple secondary antibodies to bind to a primary antibody without interfering with the hypervariable region, where an antibody binds to its target. While most secondary antibodies recognize broad isotype classes, it is possible to get secondary antibodies generated against isotype subclasses, such as mouse IgG2 or IgG4. This can be important for some applications.
Assay use
While secondary antibodies are useful signal amplifiers, the flexibility of their use varies across applications. Western blots, for instance, almost always use the indirect approach, while flow cytometry’s standard approach is the direct method. ELISAs, immunohistochemistry, and immunocytochemistry may all use either the direct approach or indirect approach, depending on the requirements of the specific assay. For the latter three, secondary antibodies are indicated when the protein of interest is found in low amounts or if the conjugated signaling molecule has a weak signal. For flow cytometry, secondary antibodies may be indicated on occasion, usually after other signal amplification techniques have been ruled out.
Ease of access
Signal amplification isn’t the only benefit of secondary antibodies. Finding the right primary antibody for your assay and protein of interest is often one of the most challenging components of any immunoimaging assay. Secondary antibodies, on the other hand, are easy to source and are readily available conjugated to a number of different signaling molecules. With the direct approach, primary antibodies must be either sourced already conjugated or conjugated in the lab. In the indirect approach, you can simply keep a stock of conjugated secondary antibodies on hand and use them to easily test different antibodies or different signaling molecules. Many labs find that a trusted stock of secondary antibodies saves time and money when developing assays.
Protocol architecture
The basic (very basic!) architecture of the indirect approach is as follows:
- Prepare the sample.
- Incubate the sample with the primary (unconjugated) antibody.
- Perform a series of wash steps to remove any unbound or excess antibody.
- Incubate the sample again with a conjugated secondary antibody, which will bind to the primary antibody.
- Perform a second series of wash steps.
- Activate the signaling molecule and perform the assay readout.
Of course, this is much easier said than done! Each technique, and sometimes each individual assay, will need to be developed and optimized for the samples, antibodies, activation, and assay readout used. You’ll note that the secondary antibodies require their own incubation and wash steps, which means that indirect methods are often significantly longer than direct ones.
Even though the secondary antibody incubation time is often shorter than that of the primary antibody, due to increased binding affinity, many indirect antibody protocols are designed to take place over multiple days. Increased run time is considered one of the major drawbacks of the indirect method, lengthening an often already lengthy process. (And while I have it on good authority that a complete Western blot can be run in a single day, I really cannot recommend it except in the most dire of circumstances.)
Multiplex assays
Mo’ proteins, mo’ problems may not be a common saying, but it probably should be. In a multiplex assay - one with multiple proteins of interest - the secondary antibody needs careful consideration before selection. If all the primary antibodies used are mouse IgG antibodies, for instance, an anti-mouse secondary will bind to all the primary antibodies indiscriminately, giving them the same output signal. In these cases, it is best to use the direct method when possible, or ensure that the primary antibodies are different species and/or isotypes. For highly multiplexed assays, using secondary antibodies which recognize specific isotype subclasses of primary antibodies can increase the possible number of target proteins.
The exception to this rule is Western blots. Instead of using signaling molecules to identify each protein of interest, this gel-based method identifies proteins solely by weight and size, relying on the signaling molecule solely asmeans of visualization. This means that if all the primary antibodies are the same isotype from the same species, only one secondary antibody will be required regardless of the number of protein targets, simplifying multiplex assays.
A brief note
Since the indirect method relies on multiple secondary antibodies attaching to a single primary antibody, it is sometimes assumed that the direct method is more quantitative than the indirect method. But in reality, multiple primary antibodies can and do attach to a single target protein, so this assumption is incorrect. With the exception of flow cytometry, most immunoimaging assays do not allow for absolute quantitative analysis of a sample. Instead, relative quantification can be done using either the direct or the indirect method.
New Methods
Time is a precious commodity in the lab and eliminating the need for secondary antibodies would save a good deal of it. Researchers are continuously looking for ways to improve direct methods and avoid the indirect ones. Some Western blots, for example, use a primary antibody labelled with a probe, eliminating the need for a secondary antibody. Other immunohistochemistry advances have found ways to increase signals from conjugated primary antibodies. The introduction of antibody fragments, such as nanobodies, has introduced new options for increasing signal strength.
For the time being, though, secondary antibodies remain the go-to option for signal amplification while providing needed flexibility in assay development. Researchers who understand how and why to use secondary antibodies will find themselves able to approach immunoimaging assays more confidently, collect a broader array of data, and occasionally ask themselves and others that most perplexing of questions: “who moved my goat anti-rabbit?!”*
*a secondary antibody generated in goat against rabbit immunoglobulins.
References and Resources
More resources on Addgene’s blog
- Antibodies 101: Introduction to Immunofluorescence
- Antibodies 101: ELISAs
- Antibodies 101: Introduction to Antibodies
Resources on Addgene.org
- Find plasmids encoding recombinant antibodies at Addgene






Leave a Comment