The blood-brain-barrier (BBB) exists to prevent substances in the blood, like proteins and viruses, from crossing into the brain. While helpful from a biological standpoint, it makes delivering AAVs to the brain difficult. Traditionally, delivery is achieved through the use of invasive methods, limiting researchers to accessible, spatially constrained regions. If you’re interested in studying the brain in a way that goes beyond such restrictions, you may want to consider a systemic capsid for your AAV.
Systemic capsids
While there is quite a bit of information available on how to use systemic capsids, when writing this post, we didn’t come across a broadly used working definition of systemic capsids. After some consideration, we offer this definition:
A systemic capsid is a capsid that has been engineered to have enhanced function after intravenous administration, distributing widely throughout the organism to target large or diffuse biological structures. Of primary interest here are systemic capsids that target the central nervous system (CNS), crossing the BBB, and those targeting the peripheral nervous system (PNS).
(Capsids, if you recall, are the outer shell of an AAV vector, which determine the vector’s tropism, including its ability to cross barriers like the BBB.)
In this post, we’ll discuss how systemic capsids are engineered, as well as highlight some considerations for their use. Then, we’ll provide information about commonly used and emerging capsids to help you decide which ones may be suitable for your experiment. Like all viral vectors, the suitability of a systemic capsid for an individual application is dependent on a number of factors, including species, strain, age, sex, area of interest, and health. While it’s always helpful to start with a literature search to see what others have done, you’ll need to experimentally test your chosen capsid before using it in an application.
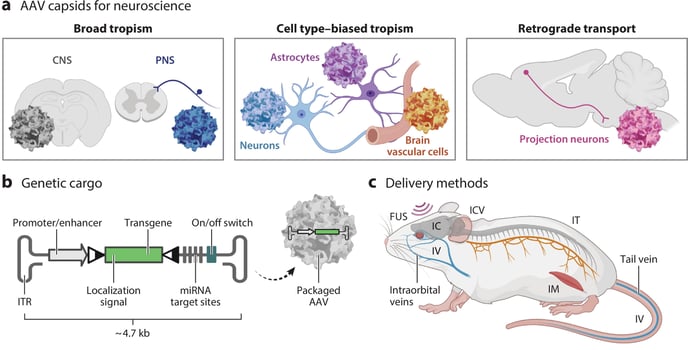
|
|
Figure 1: AAV toolkit for targeted gene delivery to the brain. The AAV (a) capsid, (b) cargo, and (c) delivery method determine cell type- or region-specific transgene expression. (a) Several engineered capsids with unique tropisms are now available. These include variants with broad CNS or PNS transduction, cell type specificity, or retrograde transport in neurons. (b) Genetic cargo can be customized to control transgene expression. Gene regulatory elements [promoters and enhancers, miRNA target sites, and recombination target sequences, denoted by triangles] facilitate tissue or cell type specificity. An on/off genetic switch can be used for expression control. (c) Viral delivery methods include focused ultrasound (FUS), intracorporeal (IC), intracerebroventricular (ICV), intramuscular (IM), intrathecal (IT), and intravenous delivery (IV). In mice, IV injections are typically administered in the lateral tail vein or retro-orbital sinus. IT injections are administered in the cisterna magna or lumbar spine. Image and caption courtesy of Challis et al., 2022. |
Where do they come from?
Systemic capsids are typically derived from AAV9, which is one of a handful of natural serotypes that can cross the BBB. However, these natural serotypes (AAV9, AAVrh10, and to a certain extent, AAV8) have low transduction efficiency in the brain along with high off-target expression, particularly in the liver, when administered intravenously.
To address these limitations, labs have developed techniques like CREATE (Deverman et al., 2016), M-CREATE (Ravindra Kumar et al., 2020), iTransduce (Hanlon et al., 2019), and DELIVER (Tabebordbar et al., 2021) to evolve new systemic capsids for both rodents and non-human primates (NHPs). These engineered capsids have increased transduction efficiency in the CNS, with greater ability to cross the BBB, or the PNS, while in many cases targeting the liver less.
Because these systemic capsids are derived from AAV9, their efficacy is generally compared to the AAV9 serotype or an engineered precursor of the systemic capsid in question (or both). Therefore, a high-efficiency systemic capsid has better efficacy than AAV9 and/or its intermediate engineered precursor.
Considering systemic capsids for your research?
While systemic capsids mostly function like your average AAVs — and have many of the same restrictions, like limited cargo space (~4.7 kb) for your genetic payload — there are a few additional, specific considerations you’ll need to be aware of before starting an experiment.
Controlling expression
As systemic capsids are… well, systemic, there’s a high likelihood of off-target expression, since they are “seeing” a great number of cells as they circulate in the blood, rather than being geographically constrained to the brain. In cases where it’s critical to minimize or eliminate off-target expression, it may be better to choose a direct delivery method over systemic delivery. Otherwise, cell-specific promoters and enhancers (Mich et al., 2021) can help restrict expression to your cell type of interest, while miRNA target sites can be used to prevent expression in cell types not of interest. Systemic capsids packaging Cre-dependent transgenes can also be paired with Cre-expressing transgenic mouse lines to restrict transgene expression to specific tissues and cell types.
If desired, spatiotemporal control of transgene expression or activity can be achieved using switches, including riboswitches and optogenetic and chemogenetic tools.
Dual expression
Promoters, miRNAs, enhancers, and switches all take up precious cargo space in AAV vectors. Dual expression systems, which use two or more AAVs to transduce the same cell, can accommodate larger, modular genetic payloads.
Since most systemic vectors are primarily derived from AAV9, the vectors have to be administered concurrently to minimize the impact of neutralizing antibodies developed in response to the first exposure to AAV9. The exception is the X1 capsid for targeting brain endothelial cells, which is available in both AAV9 and AAV1 serotypes, allowing sequential administration (Chen et al., 2023).
High viral titer
High vector genome (vg) doses are recommended for all systemic capsid applications. For example, AAV-PHP.eB is typically administered at a total dose between 1 x 1011 and 5 x 1011 vg per animal (i.e., 10 and 50 µL of vector at 1 x 1013 vg/mL titer), while AAV-PHP.S is typically used at a dose between 3 x 1011 and 1 x 1012 vg*. Most systemic capsids are easy to produce in high titer, but higher doses (>1 x 1012 vg total in mice) may increase the chances of inducing an immune response against the capsids or transgenes. Sensory neurons and liver appear especially susceptible to off-target transgene expression-induced toxicity in NHPs (Hordeaux et al, 2020; Hudry et al, 2023; Hinderer et al, 2018).
The optimal dose for each particular experiment is determined by the capsid and promoter/enhancer strengths in the cell type of interest as well as the type of transgene delivered: fluorescent proteins, optogenetic sensors, CRISPR elements, etc...
*These are only starting recommendations; you will still need to experimentally determine the best titer for your application.
 Pro tip!
Pro tip!
Want to know if a capsid is a good option for your experiment? Look it up in Addgene’s Data Hub and the CLOVER repository of published systemic AAVs to find commonly used vector cargo designs and administration doses.
Note: If capsid immunogenicity is a concern, using a purification method that removes, not just reduces, empty viral capsids in your prep can help reduce the risk of an immune response.
Strain-specific tropism in mice
Many systemic capsids express differently in different mouse strains, a phenomenon that we are calling “strain-specific tropism.” For example, AAV-PHP.B doesn’t lead to enhanced transgene expression, compared to AAV9, in BALB/cJ mice but is more effective than AAV9 in many other strains, including FVB/NCrl, 129S1/SvImJ, DBA/2J, and C57BL/6J, the strain in which the capsid was first selected.
AAV-PHP.B, and many capsids derived from it, cross the BBB through an endothelial cell protein called LY6A (Hordeaux et al., 2019; Huang et al., 2019). LY6A is functionally expressed on the surface of brain endothelial cells in C57BL/6J mice, but not in BALB/cJ mice, meaning that systemic capsids that rely on LY6A interactions cannot cross the BBB in BALB/cJ mice (Hordeaux et al., 2018). This results in strain-specific tropism (see S3 table from Huang et al, 2019 for affected mouse strains). However, other capsids cross the BBB via interactions with a similar protein called LY6C1. This protein is expressed in both BALB/cJ mice and C57BL/6J mice (Shay et al., 2023; Huang et al., 2023). Systemic capsids that cross via interactions with LY6C1 are effective in both mouse strains. It is therefore important to review the literature for use of a capsid in your strain of interest, check the mechanism of entry if known, and, as always, validate the capsid for your specific application and animal model.
Other model organisms
Tropism and efficacy can vary depending on species. LY6A and LY6C1 do not have known homologs in NHPs, and capsids that rely on interactions with those two proteins to cross the BBB are often not effective in NHPs (Liguore et al., 2019; Matsuzaki et al., 2018; Huang et al., 2023). However, several systemic capsids have been developed specifically for NHPs.
There are capsids that are effective in both NHPs and mice, but they can display species-specific tropism. For example, AAV9-X1.1 specifically transduces brain endothelial cells in mice but neurons in infant macaques (Chen et al., 2023). And these differences occur not just between mice and NHPs; some capsids show both different efficacy and tropism between NHP species, particularly between New World and Old World monkeys.
Selecting your systemic capsid
Once you’ve decided that systemic AAVs are a good fit for your experiment, it’s time to start selecting and testing systemic capsids! Figure 2 contains a decision tree for selecting a capsid, while Table 1 gives a brief overview of published descriptions of commonly used or emerging capsids. Selected capsids are further highlighted below the table.
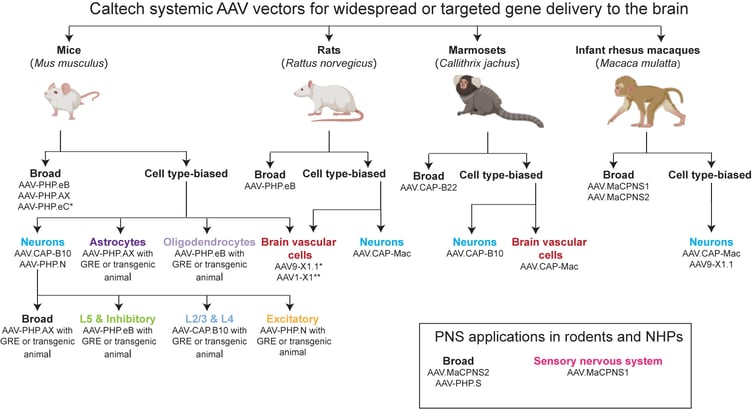
|
|
Figure 2: This decision tree can help guide your choice on which systemic capsid to use. Factors to consider include the delivery method, mouse strain, target cell type or tissue of interest, and model organism under study. *Many engineered AAVs for mice use LY6A to cross the blood-brain barrier, leading to strain-specific tropism (See S3 table (Huang, et al, 2019) for LY6A-permissive mouse strains). AAV-PHP.eC, AAV9-X1.1 and AAV1-X1 do not display strain-specific tropism and can be used in LY6A-nonpermissive mouse strains. **AAV1-X1 has not been tested in rats. For more information on reagents and protocols, visit https://clover.caltech.edu. Figure adapted from Challis et al. 2022. Image created with BioRender.com. Abbreviations: AAV, adeno-associated virus; GRE, gene regulatory element; NHP, non-human primate. |
Many of the systemic capsids in our repository are deposited by the Gradinaru lab. You can visit our Caltech Systemic Capsids page to learn more about that collection.
Table 1: Systemic capsid plasmids available from Addgene
|
Capsid |
Published Tropism |
Model Organism |
Mouse Strain-Specific Tropism? |
Reference |
|
CNS (broad) |
Mice, rats |
Yes (LY6A) |
Deverman et al., 2016 Hordeaux et al, 2019 Huang et al, 2019 |
|
|
CNS |
Mice |
Yes (LY6A) |
Deverman et al., 2016 |
|
|
CNS |
Mice |
Yes (LY6A) |
Deverman et al., 2016 |
|
|
CNS (broad): enhanced coverage for inhibitory versus excitatory neurons |
Mice, rats |
Yes (LY6A) |
Chan et al., 2017 Brown et al, 2021 Jang et al, 2023 |
|
|
PNS, including ENS (broad): somatic and visceral sensory ganglia, type II spiral ganglion neurons |
Mice, rats |
No (mechanism unknown) |
Chan et al., 2017 Asencor et al., 2022 |
|
|
CNS: neurons and astrocytes |
Mice |
No (LY6C1) |
Shay et al., 2023 |
|
|
CNS: Biased toward vasculature, but also transduces astrocytes |
Mice |
Yes (LY6A)
|
Kumar et al., 2020 |
|
|
CNS: Biased toward vasculature, but also transduces astrocytes |
Mice |
Yes (LY6A)
|
Kumar et al., 2020 |
|
|
CNS: neurons |
Mice |
Yes (LY6A) |
Kumar et al., 2020 |
|
|
CNS |
Mice |
Unknown |
Kumar et al., 2020 |
|
|
CNS: neurons, astrocytes |
Mice |
Unknown |
Jang et al., 2023 |
|
|
Brain endothelial cells (mice, rats); CNS neurons (infant rhesus macaques) |
Mice, rats, rhesus macaques |
No (mechanism unknown)
|
Chen et al., 2023 |
|
|
Brain endothelial cells |
Mice |
No (mechanism unknown)
|
Chen et al., 2023 |
|
|
Lung: ATII cells |
Mice |
N/A |
Goertsen et al., 2022 |
|
|
CNS: neurons and astrocytes |
Mice, marmosets |
Unknown |
Goertsen, Flytzanis, et al., 2022 |
|
|
CNS: balanced coverage of inhibitory and excitatory neurons |
Mice, marmosets |
Unknown |
Brown et al, 2021 Goertsen, Flytzanis, et al., 2022 Jang et al, 2023 |
|
|
CNS: neurons (rats, infant green monkey, rhesus macaque); vasculature (adult marmosets), biased toward astrocytes (adult rhesus macaque) |
Rats, marmosets, green monkeys, rhesus macaques |
N/A |
Chuapoco et al., 2023 |
|
|
CNS: neurons and astrocytes |
Mice |
No (LY6C1)
|
Hanlon et al., 2019 |
|
| AAV-BI-28 |
CNS: neurons, astrocytes, oligodendrocytes. |
Mice |
No (LY6C1)
|
Huang et al., 2023 |
|
Brain endothelial cells |
Mice, rats |
No (mechanism unknown) |
Krolak et al., 2022 |
|
|
PNS (mice and rats) CNS; PNS, including ENS (adult marmosets, infant rhesus macaques) |
Mice, rats, marmosets, rhesus macaques |
Unknown |
Chen et al., 2022 |
|
|
PNS, including ENS (mice) PNS (rats) CNS; PNS, including ENS (adult marmosets, infant rhesus macaques) |
Mice, rats, marmosets, rhesus macaques |
Unknown |
Chen et al., 2022 |
|
|
CNS: cerebrum; neuroretina |
Macaques |
N/A |
Stanton et al., 2023 |
|
|
CNS |
Mice |
No (LY6C1) |
Stanton et al., 2023 |
* indicates viral preps available. Mechanisms of crossing the BBB are indicated in parentheses in the"Mouse Strain-Specific Tropism?" column. For a complete list of mice strains with LY6A-dependent tropism, see S3 table in Huang et al, 2019.
Systemic capsids for broad cell-type coverage in rodent CNS
Many of these capsids were deposited by the Gradinaru lab. For simplicity, in each section we’ll list the Gradinaru capsids first, before moving on to deposits from other labs. AAVs not otherwise attributed were deposited by the Gradinaru lab.
AAV-PHP.B was the first systemic capsid developed and deposited by the Gradinaru lab (Deverman et al., 2016). Compared to AAV9, it effectively transduces neurons and astrocytes located in the CNS, however, it requires high doses. Requests have shifted toward the more efficient AAV-PHP.eB, developed and deposited by the same lab.
AAV-PHP.eB is the most commonly requested systemic capsid in the Addgene repository. Like its parent AAV-PHP-B, it can be produced with high viral yields relative to AAV9. At lower viral titers than AAV-PHP.B (e.g., 1 x 1011 vg/mouse), AAV-PHP.eB can transduce a greater number of neurons in the CNS (Chan et al., 2017). AAV-PHP.eB has frequently been used for screening candidate regulatory elements such as enhancers and promoters for cell type-specific targeting (Chan et al., 2017, Vormstein-Schneider et al., 2020, Graybuck et al., 2021, Lawler et al., 2022). It can also effectively transduce astrocytes or oligodendrocytes when paired with appropriate cell type-specific promoters or Cre lines. Spatial transcriptomics suggests that AAV-PHP.eB, when paired with the ubiquitous CAG promoter, is relatively biased toward L5 and inhibitory neurons and astrocytes (Fig. 4b, Jang et al., 2023). AAV-PHP.eB targets the whole mouse brain, but at low doses a regional bias towards the cortex and thalamus emerges.
AAV-PHP.eC effectively transduces neurons and astrocytes located in the CNS. It was engineered, via the M-CREATE selection method, to cross the BBB through interactions with LY6C1 and thus effectively transduces cells in the CNS of both BALB/cJ and C57BL/6J mice (Shay et al., 2023).
AAV-PHP.AX is biased toward astrocytes, but its neuron transduction encompasses more molecularly-defined neuronal subtypes and with less bias among them than AAV-PHP.eB (Fig. 3d and 4d, Jang et al., 2023). When combined with a specific promoter, AAV-PHP.AX shows greater changes in specificity than AAV-PHP.eB. For this reason, the Gradinaru lab anticipates that high-dose AAV-PHP.AX will be the capsid of choice for future promoter/enhancer screening. However, AAV-PHP.AX packages viral genomes at a lower efficiency than AAV9 and thus produces lower viral yields.
AAV-F, derived from AAV9 using iTransduce, shows tropism toward astrocytes and neurons, with greatly increased efficacy compared to AAV9 (Hanlon et al., 2019). This capsid was developed and deposited by the Maguire lab. In mice, it is sex-independent and does not show significant differences in transduction efficiency between C57BL/6J and BALB/cJ mice due to its LY6C1 dependence.
MDV1A and MDV1B were generated by the Sabeti lab using the DELIVER (Tabebordbar et al., 2021) method, selecting for variants that were effective in C57BL/6J mice, BALB/cJ mice, and cynomolgus macaques. MVD1A is effective in transducing brain cells and spinal cords of both C57BL/6J and BALB/cJ mice through LY6C1, with relatively even distribution of target gene expression (EGFP, in the depositing manuscript) across the brain.
AAV-BI-28 has been tested in C57BL/6J mice and effectively transduces NeuN+ neurons and astrocytes (Huang et al., 2023). It was rationally engineered and selected for its ability to bind to the LY6C1 protein in order to reduce strain-specific tropism in mice. It, and other AAV-BI capsids, were developed and deposited by the Deverman lab.
Systemic capsids biased toward neurons in rodent CNS
AAV.CAP-B10 was derived via M-CREATE from AAV-PHP.eB and is highly specific for neurons, with less bias toward inhibitory neurons than AAV-PHP.eB (Fig. 4c, Jang et al., 2023; Brown et al., 2021.) It has a similar transduction rate to AAV-PHP.eB in the brain, and a lower transduction rate in the spinal cord. Additionally, AAV.CAP-B10 is targeted away from the liver and shows decreased transduction in off-target organs (Goertsen, Flytzanis, et al., 2022).
AAV-PHP.N specifically transduces NeuN+ neurons, with transduction efficiency varying from 10-70% in different regions of the brain (Ravindra Kumar et al., 2020). Though less effective than AAV-PHP.eB, specificity of transduction with AAV-PHP.N is quite pronounced, even when using a ubiquitous CAG promoter. Spatial transcriptomics shows that its tropism is relatively more biased toward excitatory neurons than AAV-PHP.eB (Fig. 4c, Jang et al., 2023).
Systemic capsids biased toward endothelial cells in rodent CNS
AAV-PHP.V1 shows higher transduction in CNS endothelial cells (60%) and astrocytes (60%) than AAV9 (Ravindra Kumar et al., 2020).
AAV9-X1.1 is evolved from AAV9 and transduces GLUT1+ (endothelial) brain cells, with a higher efficiency (82 — 85% across the brain in mice) and greater specificity than AAV-PHP.V1 (Chen et al., 2023). It effectively transduces BALB/cJ, CBA/J, and FVB/NJ mice, with minimal liver transduction observed in both BALB/cJ and CBA/J mice.
AAV1-X1 was developed by transferring the 7-mer peptide region from variable region VIII of AAV9-X1.1 to that of AAV1. AAV1-X1 reproduces AAV9-X1.1’s brain endothelial cell-specific tropism, though with slightly reduced efficiency, and is not neutralized by pre-existing antibodies against AAV9-based vectors (and vice versa), enabling repeat dosing (Chen et al., 2023).
AAV-BI30, developed by the Deverman lab, effectively transduces brain endothelial cells in both BALB/cJ and C57BL/6 mice and rats. In mice, no variation in transduction efficiency was seen between different regions of the brain, and the capsid could be used to target endothelial cells in the retina and spinal cord (Krolak et al., 2022).
Systemic capsids for broad cell type coverage in rodent PNS
AAV-PHP.S effectively transduces neurons in the peripheral nervous system (PNS). It has been shown to transduce 80% of dorsal root ganglia (DRG) neurons, as well as neurons located in the enteric nervous system (ENS) and peripheral ganglia (Chan et al., 2017). It is one of a handful of systemic capsids that effectively transduce the PNS and ENS.
AAV.MaCPNS1 transduces the PNS more effectively than both AAV9 and AAV-PHP.S. It shows lower expression in the liver and in the CNS. It does not, however, show higher transduction efficiency in the ENS (Chen et al., 2022).
AAV.MaCPNS2 also transduces the PNS more effectively than AAV9 and AAV-PHP.S. It shows lower expression in the liver and no detectable transduction in the CNS. In the ENS, it has a mean transduction efficiency of ~17%, higher than both AAV9 and AAV-PHP.S, with a bias toward segments of the small intestine (Chen et al., 2022).
Systemic capsid for alveolar epithelial type II cell transduction in the rodent lung
AAV9.452sub.LUNG1 is an engineered capsid derived from AAV9 that effectively transduces mouse lungs, targeting alveolar epithelial type II cells (Goertsen, Goeden, et al., 2022). It was more effective than both AAV9 and AAV5, which has tropism for lung cells, and has increased overall transduction throughout the lungs. Like other engineered systemic capsids, it does express in the brain. Both AAV9 and AAV9.452sub.LUNG1 have significantly higher off-target expression in the liver than AAV5.
Systemic capsids for CNS transduction in New World monkeys
Cropped-min.jpg?width=488&height=435&name=Figure%203%20(1)Cropped-min.jpg)
|
|
Figure 3: Evolutionary tree depicting the phylogenetic relationship of common research species. Among these species, Old World Monkeys, like the rhesus macaque, are approximately more similar to humans than New World Monkeys and rodents. Image and caption adapted from Campos et al., 2023. |
AAV.CAP-Mac is a systemic capsid, developed and deposited by the Gradinaru lab. It was selected from a library screened in marmosets. AAV.CAP-Mac effectively transduces vasculature in adult marmosets (Chuapoco et al., 2023).
AAV.CAP-B10 shows similar behavior in marmosets as in mice, although the efficacy is lower overall. It is effective at transducing neurons, with a lower transduction rate in the spinal cord. It is detargeted from the liver and shows decreased transduction in off-target organs.
AAV.CAP-B22 was developed through the same process as AAV.CAP-B10 and shows high transduction levels in marmoset brain, with both neurons and astrocytes targeted. While marmoset liver transduction levels for AAV.CAP-B22 were lower than those seen with AAV9, they were highly variable and were not lower than those of AAV-PHP.eB (Goertsen et al., 2022). Note that in macaques, neither AAV.CAP-B22 nor AAV.CAP-B10 is more effective at transducing brain cells than AAV9 (Stanton et al., 2023).
Systemic capsids for CNS and PNS transduction in New World monkeys
The MaCPNS capsids transduce the CNS and PNS, including the ENS, effectively in adult marmosets (Chen et al., 2022). They were developed and deposited by the Gradinaru lab. They primarily transduce CNS neurons and astrocytes, and AAV.MaCPNS2 is slightly more effective than AAV.MaCPNS1.
Systemic capsids for CNS transduction in Old World monkeys
AAV.CAP-Mac is largely neurotropic in infant rhesus macaques and green monkeys (Chuapoco et al., 2023). However, in adult macaques, it appears to show a broader tropism with a potential bias toward astrocytes (Chuapoco et al., 2023). Additionally, AAV.CAP-Mac has a lower transduction rate than AAV9 in the liver.
AAV9-X1.1 effectively and predominantly transduces neurons across brain regions, with reduced off-target expression compared to AAV9 in infant rhesus macaques.
The AAV.PAL systemic capsids were developed and deposited by the Sabeti lab for use in cynomolgus macaques. PAL1A, PAL1B, and PAL1C are effective at transducing the macaque brain, except for the cerebellum, brain stem, and spinal cord. All PALs, including PAL1D, are detargeted from the liver, kidney, lung, and thymus, and transduce the heart and spleen at similar efficacy levels to AAV9 (Stanton et al., 2023).
PAL2, a second-generation PAL capsid, shows enhanced transduction efficacy compared to AAV9 in the cerebrum, but not the cerebellum, in macaques. It also effectively transduces the neuroretina. PAL2 detargets the liver and has increased DRG tropism compared to AAV9 (Stanton et al., 2023).
Systemic capsids for CNS and PNS transduction in Old World monkeys
AAV.MaCPNS1 and AAV.MaCPNS2 transduce the CNS, primarily neurons and astrocytes, as well as the PNS, including the ENS, in infant rhesus macaques (Chen et al., 2022). AAV.MaCPNS2 is slightly more effective than AAV.MaCPNS1 at transducing neurons and astrocytes in the brain, while AAV.MaCPNS1 transduces a higher percentage of neurons in the spinal cord compared to AAV9 and AAV.MaCPNS2.
Ex vivo macaque and human brain slices
Several of the systemic capsids mentioned above have also been tested on ex vivo brain slices obtained from adult pigtail (Macaca nemestrina) or rhesus macaque, and on human brain slices cultured in physiological conditions (Chen et al., 2023, Chuapoco et al., 2023).
Among the capsids tested, AAV9-X1.1 showed the greatest fold change in RNA and DNA levels (~25-fold and ~4-fold respectively) relative to AAV9 in the pigtail macaque brain slices. Similarly, AAV9-X1.1 outperformed the other capsids in the rhesus macaque and human brain slices. This capsid’s high transduction efficiency across species points to its potential utility in translational applications and in models such as human organoids.
Now what?
There are a lot of options for systemic capsids, but we hope this post helps you find the right one to test in your application.
If you think you’ve found a good fit (or if you need more options) we recommend you check out Caltech’s CLOVER Center’s extensive repository of published systemic AAV experiments and Addgene’s AAV Data Hub to learn more about the capsid you’re interested in. Then request your capsid (or plasmids) and let the real work begin — validation, validation, validation! (psst… don't forget to submit your validation data to our Data Hub once you’re finished!)
Good luck and happy transducing!
This post was written by Rachel Leeson, with significant contributions from Cynthia Arokiaraj. Addgene would also like to thank Viviana Gradinaru and Tim Shay for their support and input.
Resources and references
Recommended reading
Challis R.C., Ravindra Kumar S., Chen X., Goertsen D., Coughlin G.M., Hori A.M., Chuapoco M.R., Otis T.S., Miles T.F., Gradinaru V. Adeno-Associated Virus Toolkit to Target Diverse Brain Cells. Annual Review of Neuroscience. 2022 Jul 8;45:447-469. https://doi.org/10.1146/annurev-neuro-111020-100834
Challis, R.C., Ravindra Kumar, S., Chan, K.Y., Challis, C., Beadle, K., Jang, M.J., Kim, H.M., Rajendran, P.S., Tompkins, J.D., Shivkumar, K., Deverman, B.E., Gradinaru V. Systemic AAV vectors for widespread and targeted gene delivery in rodents. Nature Protocols;14, 379–414 (2019). https://doi.org/10.1038/s41596-018-0097-3
More resources on the Addgene blog
Viral Vectors 101: AAV Variables That Matter
Viral Vectors 101: An Introduction to AAV
References
Asencor, A. I., Dvoryanchikov, G., Makhoul, V., Tsoulfas, P., & Chaudhari, N. (2022). Selectively Imaging Cranial Sensory Ganglion Neurons Using AAV-PHP.S. eNeuro,9(3), ENEURO.0373-21.2022. https://doi.org/10.1523/ENEURO.0373-21.2022
Brown, D., Altermatt, M., Dobreva, T., Chen, S., Wang, A., Thomson, M., & Gradinaru, V. (2021). Deep Parallel Characterization of AAV Tropism and AAV-Mediated Transcriptional Changes via Single-Cell RNA Sequencing. Frontiers in immunology, 12, 730825. https://doi.org/10.3389/fimmu.2021.730825
Campos LJ, Arokiaraj CM, Chuapoco MR, Chen X, Goeden N, Gradinaru V, Fox AS. (2023). Advances in AAV technology for delivering genetically encoded cargo to the nonhuman primate nervous system. Current Research in Neurobiology, 4, 100086. https://doi.org/10.1016/j.crneur.2023.100086.
Challis, R. C., Ravindra Kumar, S., Chen, X., Goertsen, D., Coughlin, G. M., Hori, A. M., Chuapoco, M. R., Otis, T. S., Miles, T. F., & Gradinaru, V. (2022). Adeno-Associated Virus Toolkit to Target Diverse Brain Cells. Annual Review of Neuroscience, 45(1), 447–469. https://doi.org/10.1146/annurev-neuro-111020-100834
Chan, K. Y., Jang, M. J., Yoo, B. B., Greenbaum, A., Ravi, N., Wu, W. L., Sánchez-Guardado, L., Lois, C., Mazmanian, S. K., Deverman, B. E., & Gradinaru, V. (2017). Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nature Neuroscience, 20(8), 1172–1179. https://doi.org/10.1038/nn.4593
Chen, X., Ravindra Kumar, S., Adams, C. D., Yang, D., Wang, T., Wolfe, D. A., Arokiaraj, C. M., Ngo, V., Campos, L. J., Griffiths, J. A., Ichiki, T., Mazmanian, S. K., Osborne, P. B., Keast, J. R., Miller, C. T., Fox, A. S., Chiu, I. M., & Gradinaru, V. (2022). Engineered AAVs for non-invasive gene delivery to rodent and non-human primate nervous systems. Neuron, 110(14), 2242-2257.e6. https://doi.org/10.1016/j.neuron.2022.05.003
Chen, X., Wolfe, D. A., Bindu, D. S., Zhang, M., Taskin, N., Goertsen, D., Shay, T. F., Sullivan, E. E., Huang, S.-F., Ravindra Kumar, S., Arokiaraj, C. M., Plattner, V. M., Campos, L. J., Mich, J. K., Monet, D., Ngo, V., Ding, X., Omstead, V., Weed, N., … Gradinaru, V. (2023). Functional gene delivery to and across brain vasculature of systemic AAVs with endothelial-specific tropism in rodents and broad tropism in primates. Nature Communications, 14(1), 3345. https://doi.org/10.1038/s41467-023-38582-7
Chuapoco, M. R., Flytzanis, N. C., Goeden, N., Christopher Octeau, J., Roxas, K. M., Chan, K. Y., Scherrer, J., Winchester, J., Blackburn, R. J., Campos, L. J., Man, K. N. M., Sun, J., Chen, X., Lefevre, A., Singh, V. P., Arokiaraj, C. M., Shay, T. F., Vendemiatti, J., Jang, M. J., … Gradinaru, V. (2023). Adeno-associated viral vectors for functional intravenous gene transfer throughout the non-human primate brain. Nature Nanotechnology, 1–11. https://doi.org/10.1038/s41565-023-01419-x
Deverman, B. E., Pravdo, P. L., Simpson, B. P., Kumar, S. R., Chan, K. Y., Banerjee, A., Wu, W.-L., Yang, B., Huber, N., Pasca, S. P., & Gradinaru, V. (2016). Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nature Biotechnology, 34(2), 204–209. https://doi.org/10.1038/nbt.3440
Goertsen, D., Flytzanis, N. C., Goeden, N., Chuapoco, M. R., Cummins, A., Chen, Y., Fan, Y., Zhang, Q., Sharma, J., Duan, Y., Wang, L., Feng, G., Chen, Y., Ip, N. Y., Pickel, J., & Gradinaru, V. (2022). AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nature Neuroscience, 25(1), 106–115. https://doi.org/10.1038/s41593-021-00969-4
Goertsen, D., Goeden, N., Flytzanis, N. C., & Gradinaru, V. (2022). Targeting the lung epithelium after intravenous delivery by directed evolution of underexplored sites on the AAV capsid. Molecular Therapy - Methods & Clinical Development, 26, 331–342. https://doi.org/10.1016/j.omtm.2022.07.010
Graybuck, L. T., Daigle, T. L., Sedeño-Cortés, A. E., Walker, M., Kalmbach, B., Lenz, G. H., Morin, E., Nguyen, T. N., Garren, E., Bendrick, J. L., Kim, T. K., Zhou, T., Mortrud, M., Yao, S., Siverts, L. A., Larsen, R., Gore, B. B., Szelenyi, E. R., Trader, C., Balaram, P., … Tasic, B. (2021). Enhancer viruses for combinatorial cell-subclass-specific labeling. Neuron,109(9), 1449–1464.e13. https://doi.org/10.1016/j.neuron.2021.03.011
Hanlon, K. S., Meltzer, J. C., Buzhdygan, T., Cheng, M. J., Sena-Esteves, M., Bennett, R. E., Sullivan, T. P., Razmpour, R., Gong, Y., Ng, C., Nammour, J., Maiz, D., Dujardin, S., Ramirez, S. H., Hudry, E., & Maguire, C. A. (2019). Selection of an Efficient AAV Vector for Robust CNS Transgene Expression. Molecular Therapy - Methods & Clinical Development, 15, 320–332. https://doi.org/10.1016/j.omtm.2019.10.007
Hordeaux, J., Wang, Q., Katz, N., Buza, E. L., Bell, P., & Wilson, J. M. (2018). The Neurotropic Properties of AAV-PHP.B Are Limited to C57BL/6J Mice. Molecular Therapy, 26(3), 664–668. https://doi.org/10.1016/j.ymthe.2018.01.018
Hordeaux, J., Yuan, Y., Clark, P. M., Wang, Q., Martino, R. A., Sims, J. J., Bell, P., Raymond, A., Stanford, W. L., & Wilson, J. M. (2019). The GPI-Linked Protein LY6A Drives AAV-PHP.B Transport across the Blood-Brain Barrier. Molecular Therapy, 27(5), 912–921. https://doi.org/10.1016/j.ymthe.2019.02.013
Huang, Q., Chan, K. Y., Tobey, I. G., Chan, Y. A., Poterba, T., Boutros, C. L., Balazs, A. B., Daneman, R., Bloom, J. M., Seed, C., & Deverman, B. E. (2019). Delivering genes across the blood-brain barrier: LY6A, a novel cellular receptor for AAV-PHP.B capsids. PloS One, 14(11), e0225206. https://doi.org/10.1371/journal.pone.0225206
Huang, Q., Chen, A. T., Chan, K. Y., Sorensen, H., Barry, A. J., Azari, B., Zheng, Q., Beddow, T., Zhao, B., Tobey, I. G., Moncada-Reid, C., Eid, F.-E., Walkey, C. J., Ljungberg, M. C., Lagor, W. R., Heaney, J. D., Chan, Y. A., & Deverman, B. E. (2023). Targeting AAV vectors to the central nervous system by engineering capsid–receptor interactions that enable crossing of the blood–brain barrier. PLoS Biology, 21(7), e3002112. https://doi.org/10.1371/journal.pbio.3002112
Jang, M. J., Coughlin, G. M., Jackson, C. R., Chen, X., Chuapoco, M. R., Vendemiatti, J. L., Wang, A. Z., & Gradinaru, V. (2023). Spatial transcriptomics for profiling the tropism of viral vectors in tissues. Nature Biotechnology. https://doi.org/10.1038/s41587-022-01648-w
Krolak, T., Chan, K. Y., Kaplan, L., Huang, Q., Wu, J., Zheng, Q., Kozareva, V., Beddow, T., Tobey, I. G., Pacouret, S., Chen, A. T., Chan, Y. A., Ryvkin, D., Gu, C., & Deverman, B. E. (2022). A high-efficiency AAV for endothelial cell transduction throughout the central nervous system. Nature Cardiovascular Research, 1(4), Article 4. https://doi.org/10.1038/s44161-022-00046-4
Kumar, S. R., Miles, T. F., Chen, X., Brown, D., Dobreva, T., Huang, Q., Ding, X., Luo, Y., Einarsson, P. H., Greenbaum, A., Jang, M. J., Deverman, B. E., & Gradinaru, V. (2020). Multiplexed Cre-dependent selection yields systemic AAVs for targeting distinct brain cell types. Nature Methods, 17(5), 541–550. https://doi.org/10.1038/s41592-020-0799-7
Lawler, A. J., Ramamurthy, E., Brown, A. R., Shin, N., Kim, Y., Toong, N., Kaplow, I. M., Wirthlin, M., Zhang, X., Phan, B. N., Fox, G. A., Wade, K., He, J., Ozturk, B. E., Byrne, L. C., Stauffer, W. R., Fish, K. N., & Pfenning, A. R. (2022). Machine learning sequence prioritization for cell type-specific enhancer design. eLife,11, e69571. https://doi.org/10.7554/eLife.69571
Liguore, W. A., Domire, J. S., Button, D., Wang, Y., Dufour, B. D., Srinivasan, S., & McBride, J. L. (2019). AAV-PHP.B Administration Results in a Differential Pattern of CNS Biodistribution in Non-human Primates Compared with Mice. Molecular Therapy, 27(11), 2018–2037. https://doi.org/10.1016/j.ymthe.2019.07.017
Matsuzaki, Y., Konno, A., Mochizuki, R., Shinohara, Y., Nitta, K., Okada, Y., & Hirai, H. (2018). Intravenous administration of the adeno-associated virus-PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain. Neuroscience Letters, 665, 182–188. https://doi.org/10.1016/j.neulet.2017.11.049
Mich, J. K., Graybuck, L. T., Hess, E. E., Mahoney, J. T., Kojima, Y., Ding, Y., Somasundaram, S., Miller, J. A., Kalmbach, B. E., Radaelli, C., Gore, B. B., Weed, N., Omstead, V., Bishaw, Y., Shapovalova, N. V., Martinez, R. A., Fong, O., Yao, S., Mortrud, M., … Levi, B. P. (2021). Functional enhancer elements drive subclass-selective expression from mouse to primate neocortex. Cell Reports, 34(13). https://doi.org/10.1016/j.celrep.2021.108754
Ravindra Kumar, S., Miles, T. F., Chen, X., Brown, D., Dobreva, T., Huang, Q., Ding, X., Luo, Y., Einarsson, P. H., Greenbaum, A., Jang, M. J., Deverman, B. E., & Gradinaru, V. (2020). Multiplexed Cre-dependent selection yields systemic AAVs for targeting distinct brain cell types. Nature Methods, 17(5), 541–550. https://doi.org/10.1038/s41592-020-0799-7
Shay, T. F., Sullivan, E. E., Ding, X., Chen, X., Ravindra Kumar, S., Goertsen, D., Brown, D., Crosby, A., Vielmetter, J., Borsos, M., Wolfe, D. A., Lam, A. W., & Gradinaru, V. (2023). Primate-conserved carbonic anhydrase IV and murine-restricted LY6C1 enable blood-brain barrier crossing by engineered viral vectors. Science Advances, 9(16), eadg6618. https://doi.org/10.1126/sciadv.adg6618
Stanton, A. C., Lagerborg, K. A., Tellez, L., Krunnfusz, A., King, E. M., Ye, S., Solomon, I. H., Tabebordbar, M., & Sabeti, P. C. (2023). Systemic administration of novel engineered AAV capsids facilitates enhanced transgene expression in the macaque CNS. Med, 4(1), 31-50.e8. https://doi.org/10.1016/j.medj.2022.11.002
Tabebordbar, M., Lagerborg, K. A., Stanton, A., King, E. M., Ye, S., Tellez, L., Krunnfusz, A., Tavakoli, S., Widrick, J. J., Messemer, K. A., Troiano, E. C., Moghadaszadeh, B., Peacker, B. L., Leacock, K. A., Horwitz, N., Beggs, A. H., Wagers, A. J., & Sabeti, P. C. (2021). Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell, 184(19), 4919-4938.e22. https://doi.org/10.1016/j.cell.2021.08.028
Vormstein-Schneider, D., Lin, J. D., Pelkey, K. A., Chittajallu, R., Guo, B., Arias-Garcia, M. A., Allaway, K., Sakopoulos, S., Schneider, G., Stevenson, O., Vergara, J., Sharma, J., Zhang, Q., Franken, T. P., Smith, J., Ibrahim, L. A., Mastro, K. J., Sabri, E., Huang, S., Favuzzi, E., … Dimidschstein, J. (2020). Viral manipulation of functionally distinct interneurons in mice, non-human primates and humans.Nature neuroscience, 23(12), 1629–1636. https://doi.org/10.1038/s41593-020-0692-9
Topics: Viral Vectors, Viral Vectors 101, AAV





Leave a Comment