We often think about the brain’s function in terms of its regions. But equally important is the way the brain connects across these regions, a process driven by neurons. By studying how neurons are physically connected, we can begin to understand how the brain works. It’s kind of like “the head bone’s connected to the neck bone,” but with about a hundred billion neurons and six hundred trillion connections (synapses.)
In this post we’ll describe the AAV retrograde serotype (AAVrg) and how it enables scientists to access neurons, and study their connections, in a powerful way.
Using brain structure to understand brain function
So far scientists have identified 180 brain regions. Both this geography and the way the brain is connected to itself across these regions is important for understanding how the brain functions. The basis for these connections in the brain are (drum roll) neurons! Neurons are really cool because they can transmit information, for example, from one of the 180 regions to another or from the brain to the rest of the body. Therefore, by simply looking at how neurons are physically connected we can begin to understand how the brain works.
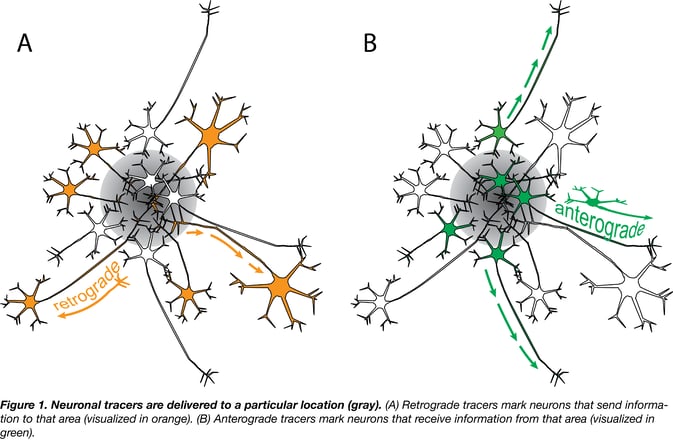
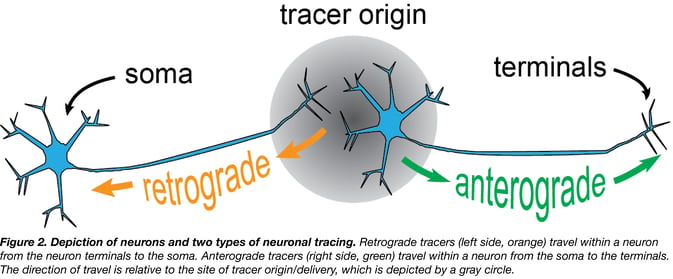
There are two methods of neuronal tracing (Fig. 1): one to visualize neurons that send information to a region (retrograde tracer, Fig. 1A), and another to visualize neurons that receive information from a region (anterograde tracer, Fig. 1B). Tracers are typically delivered to a particular location and they either trace downstream from the soma (neuron cell body) toward the axon (anterograde tracer) or upstream from the axon terminals to the soma (retrograde tracer) (Fig. 2).
Viruses as neuronal scalpels
Viruses can be injected into the brain and efficiently deliver genetic information or tools, making them useful tracers, but finding a virus that works well for retrograde tracing can be difficult. The in vivo workhorse adeno associated virus (AAV) shows relatively low retrograde transport efficiency in most serotypes. Some viruses (e.g., rabies) naturally exhibit retrograde transport and can therefore perform retrograde tracing, but often at the cost of toxicity, low expression, or poor scalability.
Scientists at Janelia Research Campus and the University of California, Berkeley engineered an AAV variant that efficiently infects neurons, directs high-level transgene expression, and has retrograde functionality to monitor and map neurons (Tervo et al., 2016). With AAVrg , scientists can functionally dissect specific neurons within their natural environment, all while reciting their favorite slogan, “look how cool the brain is!"
Using retrograde AAV
AAVrg can be used to deliver a wide array of genetic tools for neural interrogation like optogenetic tools, chemogenetic tools, and biosensors . Thus, you can map what regions are signaling to a particular site while also activating or inhibiting gene expression, detecting neuronal signaling, or manipulating any other genetically controlled function.
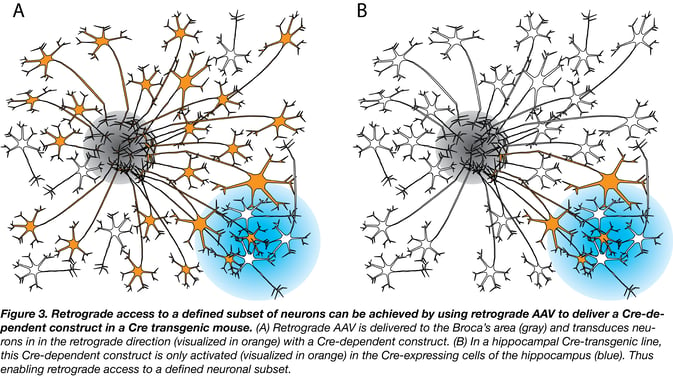
By using AAVrg in Cre transgenic mouse lines, you can access and manipulate an even further defined population of neurons. For example, say I wanted to access all the neurons that signaled from the hippocampus to Broca’s area. This could be done by injecting a Cre-dependent AAV into Broca’s area of a mouse specifically expressing Cre in the hippocampus (Fig. 3). The Cre-dependent AAV would access the neurons (Fig. 3A) that project to Broca’s area from other regions of the brain. However, the Cre-dependent AAV cargo would only become activated in the presence of Cre recombinase, which is limited to the hippocampus. Thus, only neurons signaling from the hippocampus to Broca’s area would be transduced (Fig 3B). These specific neurons could then be manipulated (e.g., activated or inhibited) by any genetic tool delivered by the AAV.
AAVrg requires high titers for efficient retrograde transport. For recombinase-dependent vectors, this can cause challenges for the experimental design as these vectors can exhibit spurious expression, particularly at the injection site (Fischer, KB. 2019). The higher the titer, the more likely you are to see spurious expression.
AAV retrograde
Unlike traditional dye-based neuronal tracing reagents, AAV particles require receptor-mediated entry into the cell. This means that not all neurons and pathways will be transduced equally well by AAV-retrograde serotypes (Skorput, 2022, Albaugh, 2020, Tervo, 2016). In fact, some neuronal pathways may not be transduced at all by this serotype. Nichole Beebe in Brett Schofield’s lab demonstrated that pathways projecting to the inferior colliculus (IC) are differentially transduced when using the retrograde serotype in both mice and guinea pigs (work you can find in AAV Data Hub!) The cortical pathways to the IC were well transduced by AAVrg while other pathways such as those in the brainstem were not. Others have noted a lack of transduction in catecholamine pathways from the spinal cord (Wang, 2018, Metcalfe, 2022) and substantia nigra (Tervo, 2016). Discrepancies in transduction efficiency can have a major impact on experimental design and interpretation of results. Take extra care when evaluating how this serotype works in your system.
Chemogenetic vectors
Expression of retrograde serotype vectors is present at both the injection site and in the putative projection neurons. When using this serotype to conduct experiments with chemogenetic vectors, this property must be taken into account since activation with chemogenetic specific ligands activates both neurons at the injection site and any projection neurons retrogradely transduced by the vector. One method for minimizing the impact of this phenomenon is to use a retrograde recombinase-expressing vector (e.g. Cre or FLP) to transduce the projection pathway neurons and then inject a recombinase-dependent chemogenetic vector directly into the region where the cell bodies of the projection neurons reside.
Impact for scientists
While the mechanisms by which retrograde AAV work are not yet understood, one thing is clear: this application opens a lot of doors in neuroscience. The retrograde serotype also has promising therapeutic applications; it can enhance therapeutic delivery across broader physical areas of the brain and enable access to neurons that are physically blocked, such as those in the spinal cord.
Now that you understand this new tool, go forth and trace some neurons!
This post was originally written by Leila Haery in 2017 and significantly updated in 2023 by Jason Nasse.
References
1. Tervo, D. Gowanlock R., et al. "A designer AAV variant permits efficient retrograde access to projection neurons." Neuron 92.2 (2016): 372-382. PubMed PMID: 27720486.
Additional Resources on the Addgene Blog
- Get Started with AAV
- Learn more about Lentivirus
- Find out More about Addgene's Viral Service
Resources on Addgene.org
- Visit Our AAV Guide Page
- Find Ready-to-use Virus for Your Research
- Visit Our Lentivirus Guide Page
Topics: Viral Vectors, Viral Vectors 101, Cell Tracing, AAV, Neuroscience







Leave a Comment