 Lentiviral vectors are one of the most popular and useful viral vectors in the lab. Advantages of lentivirus include a large genetic capacity and the ability to transduce both dividing and non-dividing cells. Lentiviral vectors are the vector of choice for many CRISPR applications, and they’ve also had success in clinical gene therapy applications. Read on to learn more about the current (and future) applications of lentiviral vectors!
Lentiviral vectors are one of the most popular and useful viral vectors in the lab. Advantages of lentivirus include a large genetic capacity and the ability to transduce both dividing and non-dividing cells. Lentiviral vectors are the vector of choice for many CRISPR applications, and they’ve also had success in clinical gene therapy applications. Read on to learn more about the current (and future) applications of lentiviral vectors!

Lentiviral vector history and components
 In the early 1990s, researchers developed viral vector systems based on retroviruses like Moloney murine leukemia virus (MMLV). The vectors could integrate into the genome, permitting long-term transgene expression, but they could only infect actively dividing cells. Another type of vector, based on adenovirus, could infect non-dividing cells, but without sustained transgene expression. To design a viral vector system that could do both, Addgene depositor Didier Trono and collaborators turned to the lentivirus HIV-1, well-known to infect non-dividing cells.
In the early 1990s, researchers developed viral vector systems based on retroviruses like Moloney murine leukemia virus (MMLV). The vectors could integrate into the genome, permitting long-term transgene expression, but they could only infect actively dividing cells. Another type of vector, based on adenovirus, could infect non-dividing cells, but without sustained transgene expression. To design a viral vector system that could do both, Addgene depositor Didier Trono and collaborators turned to the lentivirus HIV-1, well-known to infect non-dividing cells.
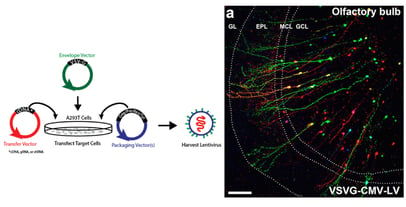
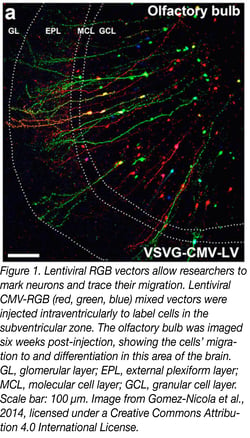
The first lentiviral vector system consisted of three plasmids: the packaging, envelope, and transfer plasmids. The packaging plasmid carried a mutated HIV-1 provirus that couldn’t package itself due to a few missing proteins. The envelope plasmid contained a viral envelope to dictate the tropism, or types of cells the vector could infect. Finally, the transfer plasmid encoded the desired transgene flanked by HIV-1 long terminal repeats (LTRs) that facilitate viral packaging and host genome integration. After co-transfection of these plasmids, 293T cells released transgene-containing lentiviral particles into the media, which could be collected for experimental use. In 1996, Naldini et al. transduced rat neurons in vivo with beta-gal and other reporters, and they showed that expression persisted for at least thirty days. Although adeno-associated viral vectors can also target non-dividing cells, lentiviral vectors can carry much more genetic cargo (8 kb vs 4.5 kb), and they remain popular for targeting and tracing cells in the brain (Figure 1).
2nd generation lentiviral packaging system
The graphic below shows how the lentiviral genome was condensed to create the 2nd-generation lentiviral system (Figure 2). The HIV genes that do remain are very important for viral production: Gag (structural precursor protein), Pol (polymerase), Tat (viral transactivator for transcriptional activation from the 5’ LTR) and Rev (facilitates nuclear export of transcripts). A different viral envelope protein (Env), usually from VSV-G due to wide infectivity, is often used as a substitute for HIV-1 Env, which can only infect CD4+ cells.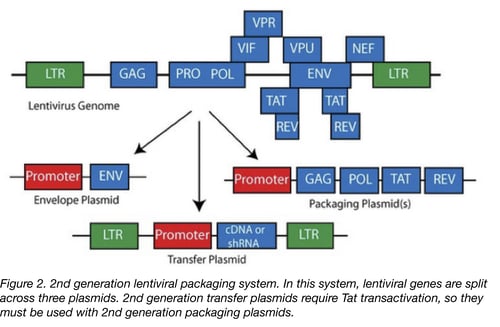
3rd generation lentiviral packaging system
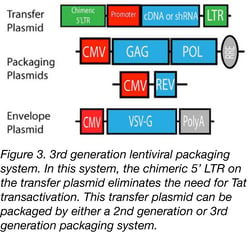 Although the 2nd generation system is safer than the original system, the possibility of creating a replication-competent virus via recombination between the transfer and packaging plasmids does exist. To further reduce this possibility and enhance biosafety, Dull et al. created the 3rd generation system (Figure 3), which differs from the 2nd generation in two key ways. First, the packaging system is split into two plasmids: one encoding Rev and one encoding Gag and Pol, increasing the number of recombination events necessary to create a replication-competent virus. Second, Tat is eliminated through the addition of a chimeric 5’ LTR containing a Tat-independent promoter. Although safer, this system may be more cumbersome to use and lead to lower viral titers due to higher plasmid number. For more information on the key differences between the 2nd and 3rd generation packaging systems, see our Lentiviral Plasmid FAQs.
Although the 2nd generation system is safer than the original system, the possibility of creating a replication-competent virus via recombination between the transfer and packaging plasmids does exist. To further reduce this possibility and enhance biosafety, Dull et al. created the 3rd generation system (Figure 3), which differs from the 2nd generation in two key ways. First, the packaging system is split into two plasmids: one encoding Rev and one encoding Gag and Pol, increasing the number of recombination events necessary to create a replication-competent virus. Second, Tat is eliminated through the addition of a chimeric 5’ LTR containing a Tat-independent promoter. Although safer, this system may be more cumbersome to use and lead to lower viral titers due to higher plasmid number. For more information on the key differences between the 2nd and 3rd generation packaging systems, see our Lentiviral Plasmid FAQs.
Lentiviral vectors in the lab
Lentiviral vectors are incredibly popular in the lab - the Trono lab’s lentiviral packaging and envelope plasmids are two of Addgene’s most requested plasmids! Lentiviral particles with the VSV-G envelope have high infectivity in a wide range of cell types, so they’re ideal for work with primary cells or other difficult-to-transfect cells. Lentiviral barcoding libraries have been used for complex applications like lineage tracing in heterogeneous tumors.
Lentiviral vectors are also commonly used to make stable transgene-expressing or knockout cell lines. Many CRISPR plasmids are designed for lentiviral use, including Addgene’s top requested plasmid in 2015: lentiCRISPR v2, used both for targeted modification and genome-wide screening.
Lentiviral vectors in the clinic
Since lentiviral vectors can deliver a large amount of DNA (~8 kb) with a relatively low immune response, it should come as no surprise that researchers are interested in developing these vectors for gene therapy. In 2003, VIRxSYS began the first clinical trial with a lentiviral vector. CD4+ T-cells from patients with HIV-1 were transduced with a lentiviral vector containing an antisense sequence against the HIV-1 envelope. As of 2013, 65 patients had been treated with this therapy, which lowered viral load and did not cause severe adverse reactions. Clinical benefits of lentiviral vectors have been observed in small trials for other diseases, including the common hematopoietic disorders sickle cell anemia and beta-thalassemia. Lentiviral vectors are promising agents for cancer immunotherapy, but this research is still in the pre-clinical/early clinical stages.
Researchers are also working to make lentiviral vectors safer. Since lentiviral vectors integrate into the genome, they could promote oncogenesis by altering local gene expression. Self-inactivating (SIN) lentiviral vectors contain a deletion in the 3’ LTR that prevents aberrant activation of nearby genes. These safer vectors have become standard in gene therapy. Researchers have also developed integrase-deficient lentiviral vectors for applications that require the large genetic capacity of a lentiviral vector but for which transient transgene expression is sufficient.
Lentiviral vector technology has come a long way since the 1990s, both in terms of research impact and safety. Many of Addgene’s most requested plasmids are used with lentiviral expression systems. If you’re new to the world of lentivirus, check out our lentiviral plasmid and biosafety guides before beginning your experiment. Let us know how you use lentivirus in the comments section below!
References
1. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. Science. 1996 Apr 12;272(5259):263-7. PubMed PMID: 8602510.
2. A third-generation lentivirus vector with a conditional packaging system. Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. J Virol. 1998 Nov;72(11):8463-71. PubMed PMID: 9765382. PubMed Central PMCID: PMC110254.
3. In-vivo RGB marking and multicolour single-cell tracking in the adult brain. Gomez-Nicola D, Riecken K, Fehse B, Perry VH. Sci Rep. 2014 Dec 22;4:7520. PubMed PMID: 25531807. PubMed Central PMCID: PMC4273606.
4. Improved vectors and genome-wide libraries for CRISPR screening. Sanjana NE, Shalem O, Zhang F. Nat Methods. 2014 Aug;11(8):783-4. PubMed PMID: 25075903. PubMed Central PMCID: PMC4486245.
5. Patient monitoring and follow-up in lentiviral clinical trials. McGarrity GJ, Hoyah G, Winemiller A, Andre K, Stein D, Blick G, Greenberg RN, Kinder C, Zolopa A, Binder-Scholl G, Tebas P, June CH, Humeau LM, Rebello T. J Gene Med. 2013 Feb;15(2):78-82. PubMed PMID: 23322669.
6. Genetic treatment of a molecular disorder: gene therapy approaches to sickle cell disease. Hoban MD, Orkin SH, Bauer DE. Blood. 2016 Feb 18;127(7):839-48. PubMed PMID: 26758916.
7. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, Down J, Denaro M, Brady T, Westerman K, Cavallesco R, Gillet-Legrand B, Caccavelli L, Sgarra R, Maouche-Chrétien L, Bernaudin F, Girot R, Dorazio R, Mulder GJ, Polack A, Bank A, Soulier J, Larghero J, Kabbara N, Dalle B, Gourmel B, Socie G, Chrétien S, Cartier N, Aubourg P, Fischer A, Cornetta K, Galacteros F, Beuzard Y, Gluckman E, Bushman F, Hacein-Bey-Abina S, Leboulch P. Nature. 2010 Sep 16;467(7313):318-22. PubMed PMID: 20844535. PubMed Central PMCID: PMC3355472.
8. Lentiviral vectors in cancer immunotherapy. Oldham RA, Berinstein EM, Medin JA. Immunotherapy. 2015;7(3):271-84. PubMed PMID: 25804479.
9. Development of a self-inactivating lentivirus vector. Miyoshi H, Blömer U, Takahashi M, Gage FH, Verma IM. J Virol. 1998 Oct;72(10):8150-7. PubMed PMID: 9733856. PubMed Central PMCID: PMC110156.
10. Integrase-defective lentiviral vectors: progress and applications. Banasik MB, McCray PB Jr. Gene Ther. 2010 Feb;17(2):150-7. PubMed PMID: 19847206.
Additional Resources on the Addgene Blog
- Your Lentiviral Plasmid FAQs Answered
- Learn How Lentiviral CRISPR Libraries Enable Genome-Scale, Knockout Screening
- Read about Genome-Wide Screening Using CRISPR/Cas9
Additional Resources on Addgene.org
- Check out Our Lentivirus Guide
- Learn about Virus Biosafety
- Find Lentiviral Vectors for Your Research
Topics: Viral Vectors, Viral Vectors 101, Retroviral and Lentiviral Vectors





Leave a Comment