This post is part of our ongoing Plasmids 101 series. Plasmids 101 will provide you with an overview of general molecular biology knowledge and techniques. If you are interested in reading more, you can find the rest of the Plasmids 101 posts here.
Now that we have covered antibiotic selection here at Plasmids 101, we can talk about an even more specific method of screening your cloning reaction. Being able to select for colonies that contain your plasmid is a great start when cloning, but how about being able to choose those that contain plasmid with an insert? Blue-white selection is a widely used method to do just that! (To read about other screening strategies, check out our screening overview blog post.)
*Note: Two Popular Vectors for Blue White Screening Available at Addgene Are: pUC18 and pUC19
Let’s begin at the beginning. The well-characterized bacterial lac operon contains a gene called lacZ that encodes for the enzyme β-galactosidase. Expression of the lac operon is induced by lactose, and also by a lactose analogue, IPTG (isopropyl β-D-1-thiogalactopyranoside). (To be completely accurate, IPTG binds and inactivates the lac operon repressor, thereby allowing lac expression).
When expressed, the β-galactosidase enzyme can break down a dye-linked substrate called x-gal (5-bromo-4-chloro-3-indolyl-β-D-galacto-pyranoside) into galactose and an insoluble blue pigment (4-chloro-3-brom-indigo). So far, so good, but how does this help us screen plasmids?
Blue-white screening in the lab
Scientists discovered that deleting a section from the lacZ gene (a mutation called lacZΔM15) creates a non-functional β-galactosidase enzyme. Providing DNA encoding this section of amino acids (called the α-peptide) to a lacZΔM15-mutant bacterial cell in trans complements the mutation allowing for a functional enzyme. This process is called α-complementation.
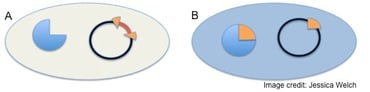 The system described above was put to practical use in the following way. Scientists engineered a multiple cloning site (MCS) into the α-peptide (represented as an orange wedge in the figure on the left) and inserted it into a plasmid, creating an α-complementation cloning vector. When a cloning reaction goes to plan and your DNA (red) is cloned into this MCS, the α-peptide gets interrupted as shown in cell A, and thus will not complement the cell's β-galactosidase mutation. An unsuccessful cloning reaction leaves the α-peptide intact, and therefore the cell will have a functional β-galactosidase enzyme through α-complementation (cell B).
The system described above was put to practical use in the following way. Scientists engineered a multiple cloning site (MCS) into the α-peptide (represented as an orange wedge in the figure on the left) and inserted it into a plasmid, creating an α-complementation cloning vector. When a cloning reaction goes to plan and your DNA (red) is cloned into this MCS, the α-peptide gets interrupted as shown in cell A, and thus will not complement the cell's β-galactosidase mutation. An unsuccessful cloning reaction leaves the α-peptide intact, and therefore the cell will have a functional β-galactosidase enzyme through α-complementation (cell B). 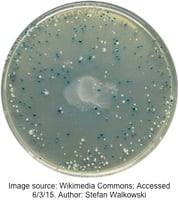
As shown on the representative plate to the right, colonies with an insert-containing plasmid have a non-functional β-galactosidase, and remain the lovely whitish-cream color of standard E. coli. On the other hand, intact β-galactosidase produces pigment from x-gal (included in the transformation plate medium), turning the bacterial colony blue. We should note that IPTG is also included in the medium to ensure transcription of the lac operon.
Tips for blue-white screening
- Use a good control: Transform the backbone plasmid without insert. All colonies on this plate should be blue, indicating that your IPTG and x-gal are working as they should be.
- Don't rush the process: It is important to give your plates enough time for any intact β-galactosidase to be expressed and process x-gal into blue pigment (16-20 hours). A plate with only white colonies is very suspicious!
- Refrigerate your plates: Placing plates at 4C for few hours after the initial overnight incubation increases pigment precipitation, enhances the blue color of negative colonies, and allows for better differentiation between blue and white colonies.
- Take care in making your plates: X-gal is light and temperature sensitive and needs to be added to media after autoclaving. If spread on top of pre-made plates, make sure it is evenly distributed and allow sufficient drying time before use.
- Beware of false positives: Blue-white screening only indicates the presence of AN insert, not necessarily YOUR insert. Any cloning artifact that disrupts the α-peptide DNA will also lead to a white colony.
- And also false negatives: These are rare, but if a small fragment is inserted in-frame, read-through can lead to a functional β-galactosidase enzyme and a blue colony. Blue-white screening is a good way to narrow down candidates for more specific analysis, like PCR or restriction digest.
- Make sure you use a proper E. coli strain (i.e. contains the lacZΔM15 mutation): XL1-Blue, DH5α, DH10B, JM109, STBL4, JM110, and Top10 are a few examples.
- Make sure you use a proper plasmid (i.e. contains the α-complementation cloning MCS): pGEM-T, pUC18 and pUC19, and pBluescript are a few common vectors.
Blue-white screening is just that – a screening process. It does not select only those cells that have taken up a plasmid and thus should be used in conjunction with selection methods. Combining selection and screening ensures that the white colonies you see are white due to successful cloning and not because the cell failed to take up the α-complementation plasmid. This way you can quickly and easily identify colonies that not only have your plasmid (antibiotic resistance), but also confirm those plasmids have your insert (blue-white screening).
Other types of screening methods
Although blue-white screening is probably the most widespread way to select for plasmids containing an insert, there are other methods. Positive selection vectors encode a gene which, when expressed, is lethal to the cell. Cloning fragments are inserted into an MCS in the center of this gene, disrupting the lethality. This is similar to α-peptide DNA disruption in the blue-white screen. Antibiotic selection is also used in conjunction with this method to ensure that positive colonies do have the plasmid containing the lethal gene. An example is the ccdB gene system commonly used in Gateway® Cloning.
There are also methods to select for plasmid-containing cells without using antibiotic resistance. These rely on cell lines susceptible to or dependent on certain media components, and are rescued by genes supplied on the transformed plasmid. Such plasmids may contain genes that allow for the use of a particular substrate in defined medium, without which the cells cannot reproduce to form colonies (used to complement auxotrophic cell lines), or genes which rescue a lethal phenotype.
Resources on the Addgene Blog
- Read Our Post on Common Lab E. coli Strains
- Learn to Use Yeast Two Hybrid Systems
- Browse All of Our Plasmids 101 Posts
Resources on the Addgene Website
- Choose the Best Molecular Cloning Technique for You
- Browse Addgene's curated list of Bacterial Expression Systems
- Find Empty Backbones for Your Experiments
Topics: Plasmids 101, Plasmids






Leave a Comment