Every few months we highlight some of the new plasmids, antibodies, and viral preps in the repository through our Hot Plasmids articles.
Here's what you'll find in this post:
- Peptide-assisted genome editing for primary cells
- Monomeric StayGold variants
- AAV.CAP-Mac for systemic gene transfer in primate brains
- Endothelial-specific AAV delivery
- Recombinant anti-FLAG tag antibodies
Peptide-assisted genome editing for primary cells
Even with the advances in CRISPR technology over the past decade, primary cells remain difficult to genetically edit due to toxicity from electroporation or poor cellular entry with other transfection methods. The Junwei Shi Lab addressed this problem by optimizing the Cas protein delivery with Peptide-Assisted Genome Editing (PAGE). They fused the Cas enzyme to cell-penetrating peptides and co-incubate it with an “assist peptide” that facilitates endosomal escape. Combining Cas9-PAGE with lentiviral sgRNA expression yielded editing of 80–90% in human cell lines and primary cells. Although cell entry of a Cas9 ribonucleoprotein (RNP) was limited (likely due to its long, negatively charged sgRNA), RNP delivery was much better with Cas12, which uses a much shorter crRNA. Cas12-RNP-PAGE achieved high editing frequency (nearly 100% in some cases) and minimal toxicity, including in clinically-relevant lines such as CAR-T cells. Plus, this method is fast! These editing frequencies can be achieved with a 30-minute incubation, even with multiplexed guides.
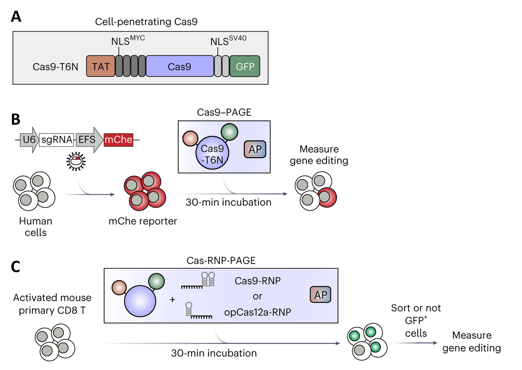 |
| Figure 1: A) Cell-penetrating Cas9, fused to HIV TAT, Myc and SV40 Nuclear Localization Signals, and GFP. B) Workflow to assess Cas9-PAGE editing with viral sgRNA targeting mCherry reporter (mChe), with co-incubation with assist peptide (AP). C) Workflow for Cas-RNP-PAGE editing of primary T cells, by co-incubation of Cas-RNP and assist peptide. Adapted from Zhang et al. 2023 with permission. |
- Zhang, Z., et al. (2023). Efficient engineering of human and mouse primary cells using peptide-assisted genome editing. Nature Biotechnology, 10.1038/s41587-023-01756-1. https://doi.org/10.1038/s41587-023-01756-1. PMID: 37095348.
Breaking up is hard to do: Monomeric StayGold variants
By Mike Lacy
In 2022, Atsushi Miyawaki and colleagues identified a highly bright and stable fluorescent protein, StayGold. Although it quickly saw wide interest, its dimeric nature can interfere with some applications. Several labs have been working to produce monomeric versions that break up the dimerization without losing that brightness and stability. Here’s a quick overview of these new variants:
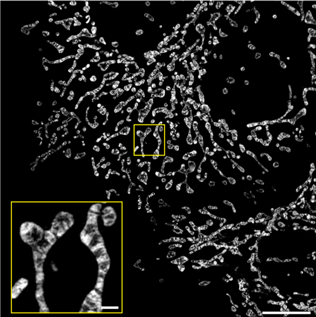
|
|
Figure 2: Inner mitochondrial membranes imaged with structured illumination microscopy in HeLa cells expressing COX8a-mStayGold. Scale bars 10 μm (main), 1 μm (inset). Image reused from Ando et al. 2023, under CC-BY license. |
The Miyawaki lab generated a series of StayGold mutants and identified a triple mutant, Y187F, R144I and T155Q, which they named QC2-6 FIQ or mStayGold (Ando et al., 2023). This variant has similar photostability and brightness compared to the original, though its maturation is even faster.
Published just a few days later, Mohan Balasubramanian and colleagues showed the single mutation E138D is sufficient to disrupt dimerization while maintaining the bright and stable fluorescence of the original (Ivorra-Molla et al., 2023). They also named their variant mStayGold.
It is worth mentioning that Kiryl Piatkevich and colleagues generated yet another monomeric StayGold variant (Piatkevich et al., 2023). Check out their preprint for more details.
Not only will these mStayGold variants be valuable tools for your next experiments, they will also likely inspire further-improved versions. Thanks to Christophe Leterrier, whose FocalPlanes piece on mStayGold inspired and informed this section.
Find mStayGold plasmids at Addgene here!
- Ando, R., et al. (2023). StayGold variants for molecular fusion and membrane-targeting applications. Nature Methods, 1–9. https://doi.org/10.1038/s41592-023-02085-6. PMID: 38036853. Preprint: https://doi.org/10.21203/rs.3.rs-2941917/v1.
- Ivorra-Molla, E., et al. (2023). A monomeric StayGold fluorescent protein. Nature Biotechnology, 1–4. https://doi.org/10.1038/s41587-023-02018-w. PMID: 38081970. Preprint: https://doi.org/10.21203/rs.3.rs-2684100/v1.
- Piatkevich, K., et al. (2023). Bright and stable monomeric fluorescent proteins derived from StayGold. ResearchSquare (Preprint). https://doi.org/10.21203/rs.3.rs-3188559/v1.
Neurogenetic alchemy: CAP-Mac unleashes the secrets of primate brains with brilliance
By Hridaya Patel (Addgene co-op)
Researchers in the Gradinaru lab have developed an engineered adeno-associated virus (AAV) vector named AAV.CAP-Mac for non-invasive systemic gene delivery in non-human primate (NHP) brains.
Systemic gene delivery capsids previously identified in rodents have not been widely successful in primates. The authors used a multispecies approach to generate and screen a library of AAV9 variants (Chuapoco et al., 2023). They found the best-performing variant, CAP-Mac, was effective at breaching the blood-brain barrier and delivering a transgene, with neuron bias observed in young macaques and vascular bias in mature marmosets. CAP-Mac also robustly expressed fluorescent reporters in green monkeys and cultured human neurons, with broader neuronal expression than AAV9. This new capsid will be a valuable tool for investigating NHP brains.
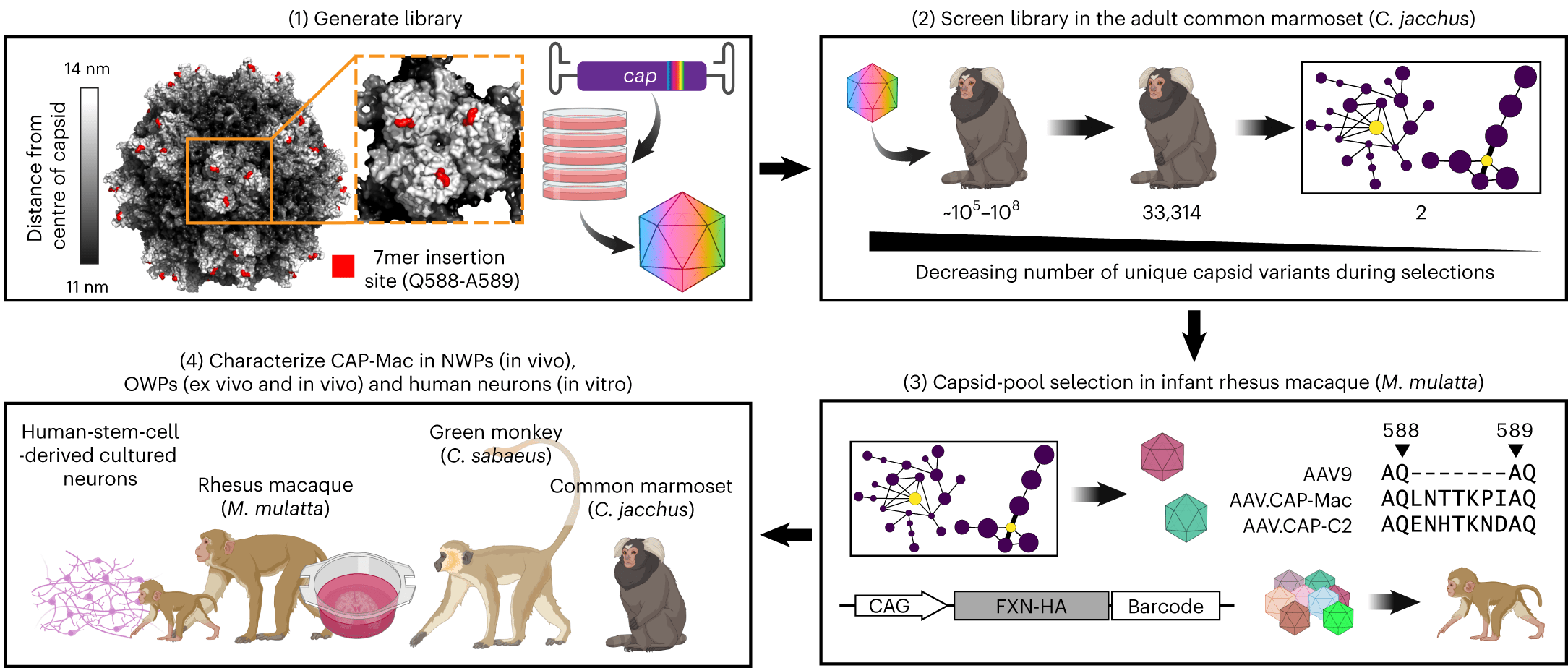 |
| Figure 3: Overview of library generation and screening that led to identification and characterization of AAV.CAP-Mac. Image reproduced from Chuapoco, Flytzanis, Goeden, et al. 2023, under CC-BY license. |
Find the AAV.CAP-Mac plasmid here!
- Chuapoco, M.R., Flytzanis, N.C., Goeden, N. et al. Adeno-associated viral vectors for functional intravenous gene transfer throughout the non-human primate brain. Nature Nanotechnology, 18, 1241–1251 (2023). https://doi.org/10.1038/s41565-023-01419-x. PMID: 37430038.
Endothelial-specific AAV delivery
By Cythina Arokiaraj (Gradinaru lab)
Another useful viral tool deposited by the Gradinaru lab, AAV1-X1, was developed by transferring the 7-mer peptide region from variable region VIII of AAV9-X1.1 to that of AAV1. This viral vector has brain endothelial cell-specific tropism, with slightly reduced efficacy compared to AA9-X1.1. Importantly, AAV1-X1 is not neutralized by pre-existing antibodies against AAV9-based vectors (and vice versa), enabling repeat dosing. In a proof-of-concept study, mouse LY6A was delivered to brain endothelial cells in mice lacking LY6A using AAV1-X1 (Chen et al., 2023); then, when AAV-PHP.eB was injected three weeks later, that LY6A transgene rescued the enhanced brain potency of AAV-PHP.eB.
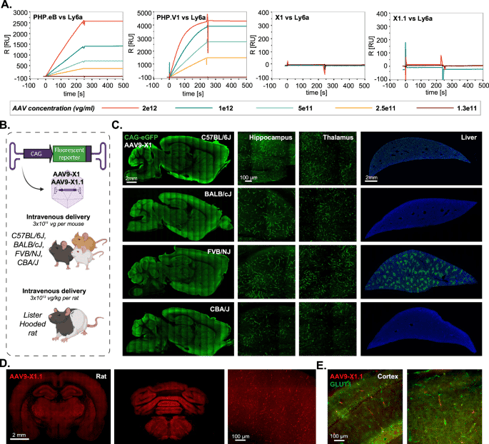 |
| Figure 4: AAV9-X1 and AAV9-X1.1 efficiently transduce brain endothelial cells across diverse mice strains and rats. Figure from Chen et al (2023) under CC-BY license. |
Chen, X., et al. (2023). Functional gene delivery to and across brain vasculature of systemic AAVs with endothelial-specific tropism in rodents and broad tropism in primates. Nature Communications, 14(1), 3345. https://doi.org/10.1038/s41467-023-38582-7. PMID: 37291094.
Recombinant anti-FLAG tag antibodies
Of all the epitope tags we know and love, the FLAG tag (amino acid sequence DYKDDDDK) may be one of the most versatile. Whether you plan to purify or image a recombinant protein, the FLAG tag has proven itself a good option for essentially all applications. Addgene’s plasmid collection includes over 10,000 plasmids with a FLAG tag (!), but did you know that we distribute ready-to-use recombinant antibodies as well? Our collection includes a mouse and a chimeric rabbit (original mouse Fab region with rabbit Fc) anti-FLAG antibody, both of which are also available in trial sizes. Addgene recommends these recombinant antibodies for use in western blots, though the original parent clone (M2) has been widely used in a variety of applications.
Topics: Hot Plasmids





Leave a Comment