The stress of finding a ‘good’ antibody is something we’ve all experienced. Finding an antibody that works for your application, specifically detects your protein, is species compatible, and doesn’t come with a high background can be a huge challenge. Epitope tags eliminate the need for target-specific antibodies and have been widely used across species and applications. In this blog, we will review the most common tags, the antibodies used to detect them, and how they can fix your antibody woes.
What are epitope tags?
Epitope tags are short peptides introduced at the N or C terminus of a protein that are bound by antibodies for the purpose of purification or detection of the tagged protein. These peptides consist of as little as 8 or as many as several hundred amino acids, depending on the tag, and are introduced via knock-in at a genomic locus or more commonly via cloning of recombinant proteins.
Why use a tag?
If you are fortunate enough to study actin, then you may not need to read this - but for many other proteins, quality antibodies do not exist, or they don’t exist for a specific application, or they exist but are unreliable for a variety of reasons (Baker, Nature). Tagging your protein allows you access to a variety of antibodies that have almost certainly already been optimized for your application. Depending on the tag, directly imaging or tracking your protein may also be possible, without the need for an antibody. Tags can enable the separation of detection of a mutant protein vs. endogenous WT levels of the same protein when both are expressed in the same system. Tagging can also aid in protein purification! In this blog, though, we are focusing on tagging for detection purposes (stay tuned for our affinity purification tag blog!).
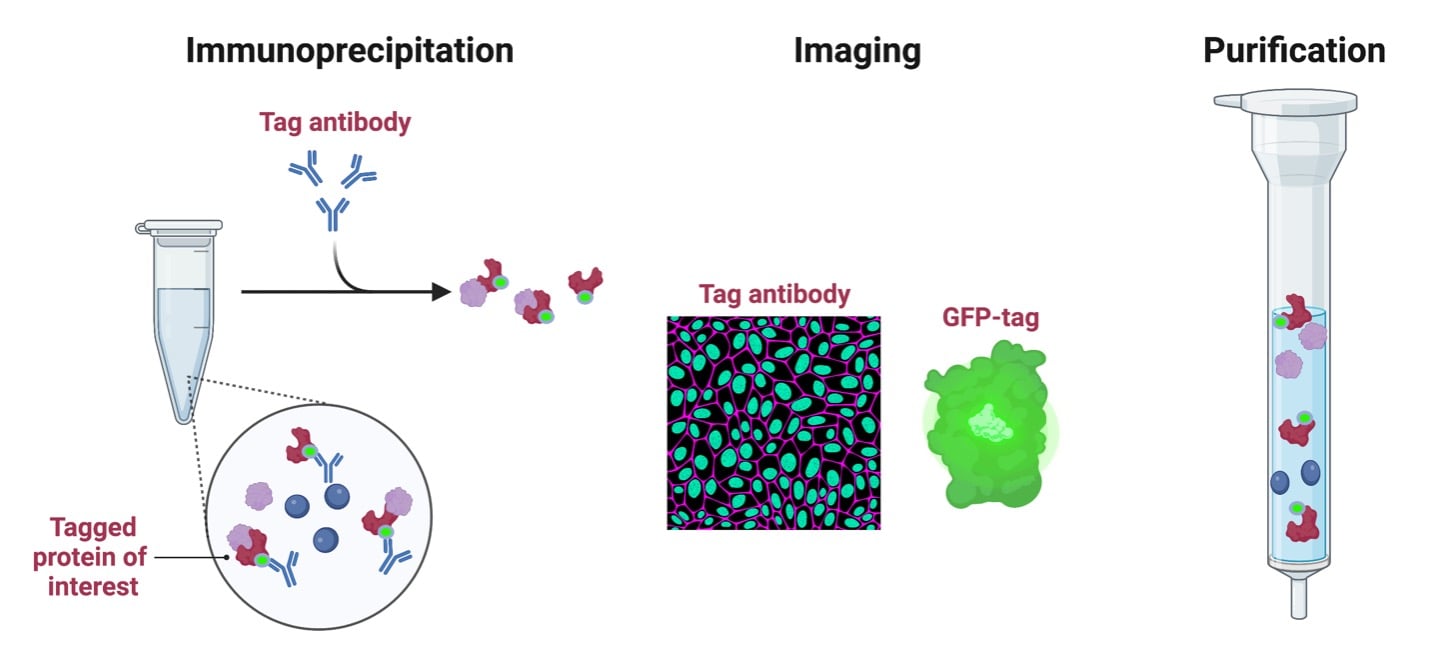
Applications of epitope tags |
The tags
A number of epitope tags exist – all with individual optimizations and specific monoclonal antibodies. Below we review the most commonly available tags and their antibodies along with some pros and cons.
FLAG
FLAG is a synthetically derived 8 amino acid tag (DYKDDDDK) that can be added multiple times (often 3X FLAG, but can be more!) to a protein’s termini to increase detection. The tag can also be removed by treating the protein with enterokinase, which recognizes the 5 amino acids on the C terminal of the tag. Of the most common tags, FLAG is the most charged, with 5 negatively charged and 2 positively charged amino acids. FLAG tag has been used with moderate to high success rates across all applications. You can find FLAG antibodies on Addgene’s catalog including a mouse monoclonal (M2) as well as a chimera (rabbit).
Myc
The Myc tag is a 10 amino acid peptide (EQKLISEEDL) derived from the protooncogene of the same name. The tag has a net negative charge with 4 negatively charged and 1 positively charged amino acids. Myc tags are frequently used for western blot, flow cytometry, and immunofluorescence . However, protein purification is not ideal with Myc, as low pH conditions are required during the purification, which may affect protein function.
Fun fact: The antibody to Myc was developed in 1985 and is still the most prevalent clone used today! Goat, chicken, mouse, or rabbit? Addgene has you covered with monoclonal antibodies to Myc for all of these species.
HA
The HA tag is a 9 amino acid peptide (YPYDVPDYA) derived from human influenza hemagglutinin. The tag has two negatively charged amino acids for an overall negative charge. This tag has been used successfully for immunoprecipitation, western blot, and immunofluorescence with some success for protein purification as well. This tag is not suitable for use in apoptotic cells as Caspase3/7 both cleave after the DVPD sequence. Check out Addgene’s monoclonal antibodies (mouse, rabbit, and chicken) to this tag!
GFP
GFP is more than a peptide – it’s a 238 amino acid protein that can also be used as a tag! GFP was originally derived from jellyfish (Aequorea victoria) and can serve as an epitope tag as well as a fluorescent marker. The GFP tag is ideal for live cell imaging, FRET, flow cytometry, or immunofluorescence where an antibody can’t be used or that step can be skipped. It has the flexibility of also being used for immunoprecipitation, purification, and western blot as monoclonal antibodies also exist for imaging. The obvious con of GFP is its size – which can possibly interfere with protein function and localization. If you need monoclonal GFP antibodies or guidance on designing a GFP tag Addgene has you covered!
Potential tag issues
Tags have a lot of major benefits – as discussed above – but there are a few instances when they should be used with caution or not at all. The introduction of a tag at the N or C terminus of a protein may disrupt the biological function of some proteins. Protein folding issues, steric hindrances, and destabilization can be brought about by the tag so it’s important to validate tagged protein functionality (Arribere, et al). In rare cases, tags can also affect protein localization and solubility, so be on the lookout for aggregation of your protein of interest!
Ready to start epitope tagging?
References and Resources
References
Terpe, K., Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol. (2003) 60:523-533 DOI: 10.1007/s00253-002-1158-6
Baker, M., Reproducibility crisis: Blame it on the antibodies. Nature (2015) 521, 274-276 DOI: 10.1038/521274a
Arribere, J. A., et al., Translation readthrough mitigation. Nature. (2016) 534, 719-723. DOI: 10.1038/nature18308
Additional resources on Addgene.org
Additional resources on the Addgene blog
Topics: Antibodies





Leave a Comment