There can be no doubt that CRISPR/Cas9 technology has been a breakthrough for the genome-editing field and the greater scientific community. In 2014, we wrote a blog post on CRISPR’s potential for correcting monogenetic diseases. Now, almost 10 years later, CRISPR’s potential for treatment is no longer an accurate descriptor; progress in clinical therapy is more fitting, so it is time to update our post.
From Mice to Men
When this article was originally written, the main focus was on editing animal models of disease and cultured cells. For example, in 2013, Jinsong Li and colleagues chose to explore CRISPR’s therapeutic potential in mice with a dominant cataract disorder caused by a single copy of a gene known as Crygc (Wu et al). They found that the cataracts phenotype could be rescued by zygote co-injection of Cas9 and a single-guide RNA targeting the mutant allele. When all was said and done, the researchers had cured 24 mice of their disease.
Meanwhile, Hans Clevers and colleagues applied CRISPR to disease correction in adult stem cells isolated from two patients with cystic fibrosis (Schwank et al). They were able to demonstrate functional correction of the gene in clonally expanded organoids.
This work, and many others, pushed the field forward to where it is today. Below we will review some of the CRISPR-based editing therapies that are currently approved or have made significant progress in clinical trials.
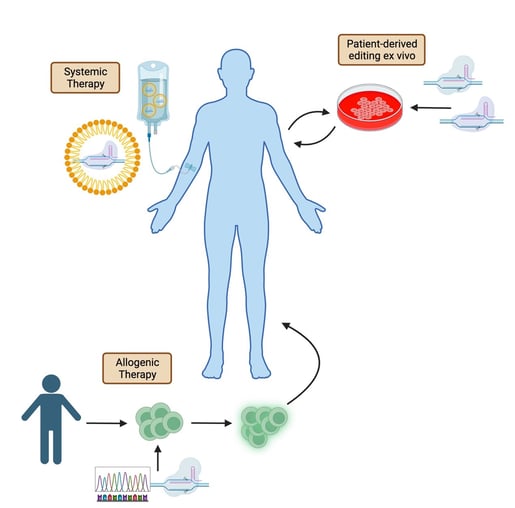
|
|
Figure 1: Types of delivery and editing strategies for CRISPR therapies. Systemic therapies involve CRISPR-Cas delivery directly to the patient. Patient-derived cells can alternatively be harvested, edited ex vivo, and then returned to the patient. Finally, allogenic therapies involve editing healthy donor-derived cells and delivering them to the patient. |
Current CRISPR Therapies
Cancer and CAR-T therapy
The FDA has already approved CAR-T immunotherapy for cancer treatment by traditional gene therapy pipelines (viral), and researchers are currently working on giving this treatment a CRISPR spin. CRISPR CAR-T treatments for leukemia, lymphoma, and some solid tumors are currently in early-phase clinical trials and have shown promising preliminary results (Dimitri et al).
CRISPR engineering of CAR-T cells is particularly promising because of its potential for allogenic “off-the-shelf” treatment. In short, instead of using the patient’s own cells that have been modified ex vivo, previously edited healthy donor cells can be delivered immediately, saving precious time. CRISPR can aid in editing these cells to avoid a negative immune response (e.g., graft vs. host) as well as the introduction of the cancer-fighting component, CAR. Clinical trials are underway to explore CRISPR’s capacity for both classes of edits in cancer therapy.
Blood disorders
Two blood disorders are promising CRISPR targets — Sickle Cell Disease (SCD) and Transfusion-dependent beta thalassemia (TBT), both of which affect hemoglobin. Most of the targeted treatment strategies for these disorders have involved turning on fetal hemoglobin to compensate for defects in the adult hemoglobin produced (Zarghamian et al). These therapies are delivered with an autologous approach, editing patient cells ex vivo and then returning them to the patient.
The first human trial involving CRISPR Cas9 for these diseases was sponsored by Vertex Pharmaceuticals and CRISPR Therapeutics. Data shared from these trials has been outstanding, with almost all patients experiencing sustained and substantial relief from both disorders. As of publication, submissions to the UK, EU, and USA federal drug approval boards are being finalized, and it is very likely the treatments will be approved for use in the next few months.
Several other companies have followed suite, with Beam Therapeutics initiating a base editor-based clinical trial for SCD in 2022 and Editas Medicine starting a Cas12-based clinical trial for SCD in 2022 as well.
Hereditary transthyretin amyloidosis (hATTR)
hATTR is caused by a variety of single nucleotide polymorphisms (SNPs) in the TTR gene and is molecularly characterized by misfolding of the TTR protein, which contributes to the disease phenotype. Intellia initiated a CRISPR therapy for hATTR with the first-ever systemic CRISPR therapy. They engineered a CRISPR-Cas construct targeting the diseased allele specifically. Early-stage trials showed a high success rate in clearing misfolded protein levels as measured in the blood. More information is expected soon on how this therapy is affecting hATTR patient outcomes.
Bacterial infection and HIV
Most CRISPR therapies have been targeted to precisely edit with the goal of ‘fixing’ mutations or introducing a desired edit, but this particular class of treatments has quite the opposite aim: to destroy.
One treatment currently in clinical trials includes Cas3 (the shredder member of the Cas family) as part of a cocktail therapy for urinary tract infections. The Cas3 enzymes target several strains of E. coli and lethally chop their genomes into many, many pieces. Locus Biosciences is currently running this trial, with more updates expected in the coming months/years.
The CRISPR-HIV therapy currently in clinical trials is somewhat similar (Hussein et al). It employs Cas9 to cut at two sites within the viral genome to excise a large portion of it. This ongoing trial is sponsored by Excision Biotherapeutics.
Additional ongoing trials
The CRISPR therapies don’t stop with the list above! There are many, many more in the earlier stages of the pipeline, ones that have experienced hiccups (either clinically or financially), and others we just don’t have the time to talk about. Other treatments currently in clinical trials include, but are not limited to, ones targeting muscular dystrophy, cardiovascular disease, inflammatory diseases, blindness, and diabetes.
Directions and obstacles for future CRISPR therapies
CRISPR technology is rapidly evolving, and many of the challenges with therapies involve patient safety, effectiveness, and delivery. Patient safety is important for allogenic therapies to prevent a graft vs. host response. Off-target effects are also important safety considerations for all CRISPR therapies, but especially concerning when using a systemic approach. Regarding effectiveness, if the editing frequency isn’t high enough to make a physiological difference, then the treatment will not work in a meaningful way. Delivery, especially for systemic approaches, has its own unique set of obstacles. Thus far, several targeted therapies have taken advantage of what our current delivery technologies are suited for. For example, lipid nanoparticle delivery tends to be trafficked to the liver. Thus, it has been used for hATTR therapy, since TTR is primarily made in the liver. Similarly, for cardiovascular disease approaches targeting cholesterol, these particles were an obvious delivery choice.
In sum, CRISPR therapies have made significant clinical strides since we first wrote this blog post. The future success of CRISPR in the clinical will rely on improving and evolving better CRISPR systems as well as improving delivery strategies to make fast, effective, and targeted therapy possible.
This post was originally written in 2014 by Kendall Morgan and updated in 2023 by Susanna Stroik.
References and Resources
References
Yuxuan Wu et al. "Correction of a Genetic Disease in Mouse Via Use of CRISPR/Cas9." Cell Stem Cell. 13, 659-662 (2013).
Gerald Schwank et al. "Functional Repair of CFTR by CRISPR/Cas9 in Intestinal Stem Cell Organoids of Cystic Fibrosis Patients." Cell Stem Cell. 13, 653-658 (2013).
Dimitri, A., Herbst, F. & Fraietta, J.A. Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Mol Cancer 21, 78 (2022). doi:10.1186/s12943-022-01559-z
Zarghamian P, Klermund J, Cathomen T. Clinical genome editing to treat sickle cell disease-A brief update. Front Med (Lausanne). 2023 Jan 9;9:1065377. doi: 10.3389/fmed.2022.1065377
Hussein M, Molina MA, Berkhout B, Herrera-Carrillo E. A CRISPR-Cas Cure for HIV/AIDS. Int J Mol Sci. 2023 Jan 13;24(2):1563. doi: 10.3390/ijms24021563
Resources on Addgene.org
Resources on the Addgene blog
Topics: CRISPR, CRISPR Therapeutic Applications





Leave a Comment