The biomedical field is often concerned with understanding the cause of diseases and how to treat those diseases. The “cause of disease” often requires understanding the disease genetics and the “treatment” usually requires drugs. While we often think of these two fields as separate, they are deeply intertwined. In this blog we will review how CRISPR technology has brought drug and genetic screens together and expedited the drug discovery pipeline.
Basics of drug discovery
Present drug discovery takes one of two approaches. The first route involves identifying a gene/pathway to develop a drug for (aka a druggable target). Diseases with defined, straightforward causes (a specific mutation in a known gene = disease onset) may be best served by this type of targeted design. Alternatively, a disease model can be generated, and drugs screened for a change in pathology. If the disease pathology is complex, a model may be most effective. Once drug candidates are isolated, they can be validated for specificity to their target, or their direct target can be identified if it isn’t known. If a strong candidate emerges, downstream optimization is performed to take the drug to the pre-clinical and human trial phases.
So how does CRISPR factor into this workflow? CRISPR both speeds up the process and can help weed out a lot of poor drugs before time and money gets put into them in those later stages.
Target identification
Not all diseases are fully characterized or have a simple pathology. In these cases, preliminary identification of potential targets by CRISPR can accelerate the drug pipeline. High-throughput CRISPR screens can be used to inhibit, activate, or knockout many genes at a time to identify ‘hits’ - genes that affect disease progression or onset. These hits can reveal individual genes or sometimes even genetic pathways that are worth investigating for drug development. Due to the difficulty of drugging some targets – the dreaded ‘undruggable’ proteins – having multiple hits or an entire pathway of possibilities greatly increases the odds of finding a quality drug downstream. In some cases, there may already exist a drug which targets some of the candidate genes!
Disease model generation
CRISPR has made the generation of genome edits accessible, fast, and easy. In cases where the mutational basis of a disease is known – repeat expansion, gene knock out, etc. – CRISPR can be used to generate cell line or animal models which have the precise disease genetics. Since CRISPR has been adapted for use in primary cells, many tissue types, and across species, it enables researchers to select and generate physiologically relevant models. In complex diseases, it can aid in generation of multiple edits or implementation of expression systems to accurately mimic a disease phenotype.
CRISPR can also be used to generate cell line knock-ins of patient mutations which are variants of unknown significance – mutations with unclear effects on disease pathology - to determine if a patient would respond to an existing therapy. In theory, CRISPR could even be used to generate patient mutations and then test both individual and combinatorial drugs on for effectiveness prior to patient treatment.
Target validation
When a target is known and a drug is developed, it’s good practice to measure the specificity of that target and validate the hit compound. The standard is to test the drug on models which are genetically proficient and deficient for the target. CRISPR has revolutionized the generation of knockout cell lines which has made this control step extremely accessible. For example, let’s say you identified gene target X for BRCA-deficient breast cancer and identified several candidate inhibitors of gene X in a screen. You generate a gene X knockout in a cancerous background and treat the parental cell line and the X knockout with your drug(s). If a drug is equally toxic to both lines, it probably has either high toxicity or high off target effects, both of which make it a non-ideal drug. If a drug selectively kills the wildtype line with minimal to no toxicity in the knockout line, it is likely a specific drug with low off-target toxicity. This added QC is where CRISPR can save time and money to make sure quality candidates are selected.
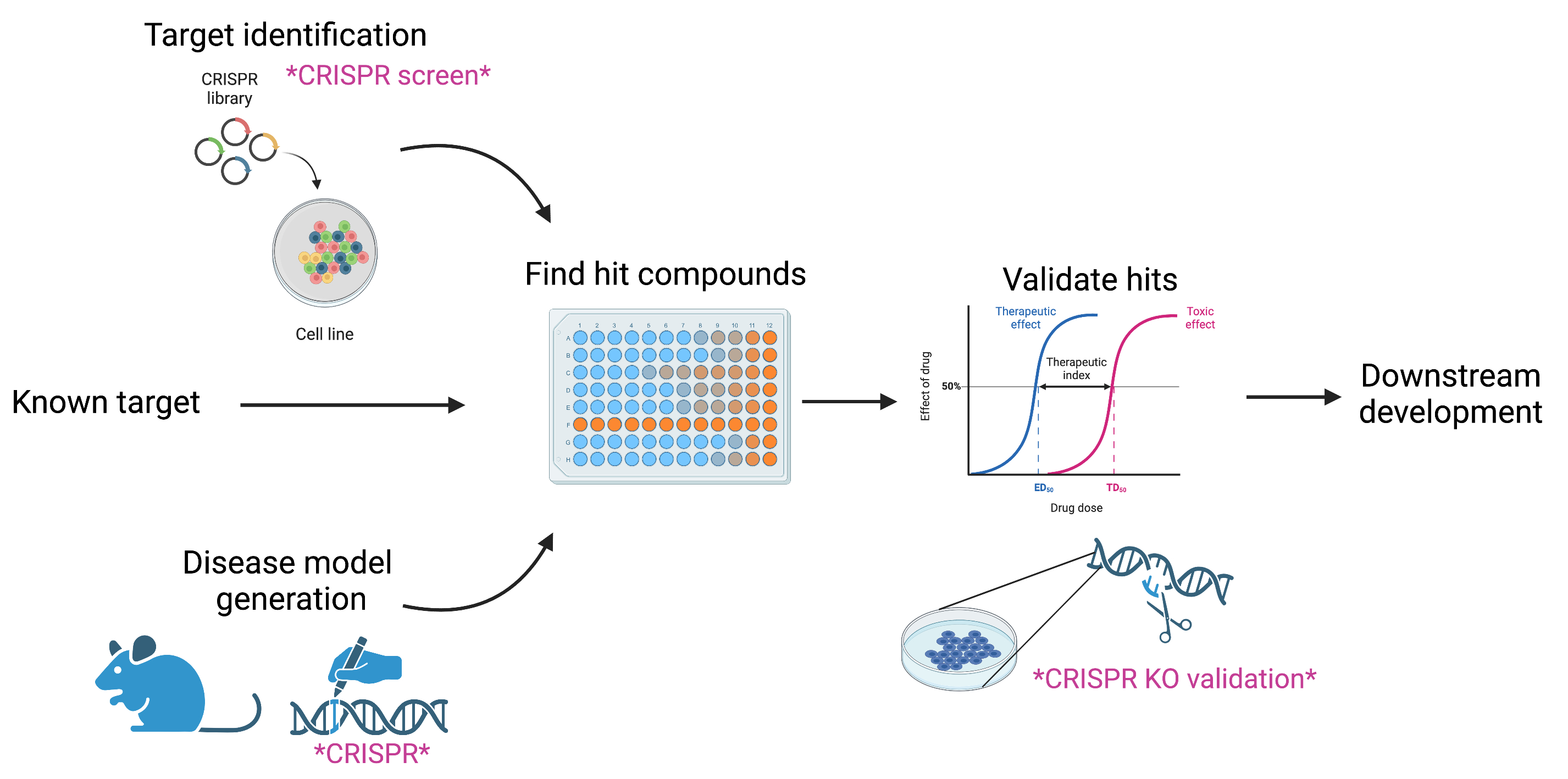
CRISPR aids in multiple stages of the drug discovery pipeline. |
CRISPR and drugs in the future
While CRISPR has already done a lot for drug discovery, there is more on the horizon. Cas enzymes have been engineered to have many unique capabilities which has advanced what type of edits can be made in the contexts outlined above. Layers of control have also been added to the CRISPR technology to allow time-controlled edits to occur, such as in the context of a specific developmental phase. As CRISPR keeps advancing, so will drug discovery – it’s an exciting time to be in the biomedical research field!
References and Resources
References
Fellmann, C., et al., Cornerstones of CRISPR-Cas in drug development and therapy. Nat Rev Drug Discov (2017) 16(2):89-100. DOI: 10.1038/nrd.2016.238
Additional resources on the Addgene blog
- CRISPR 101: Making a Knock-In Cell Line
- Using CRISPR-Cas9 to Edit Disease Out of the genome
- Tips for a first time CRISPR User (by a first time CRISPR User)
Additional resources on Addgene.org
Topics: CRISPR 101





Leave a Comment