If you are lucky in lab life, you will have a plethora of antibody options for your experiment (all well validated for your application, of course!) When the stars are aligned and the lab gods are smiling down at you, you may wonder which antibody should I pick? Do I go for the rabbit or the mouse? Is mouse IgG1 better or IgG2a? Will my choice affect my experiment? In this post we will review some of the main factors that affect isotype choice.
Target and application should be considered
The first question you may have is whether a monoclonal or polyclonal antibody is better for your experiment. Monoclonal antibodies contain a single antibody clone and therefore a single isotype while polyclonal antibodies contain a mix of different isotypes and subclasses. They each have their own advantages. Since monoclonal antibodies contain a single clone they have higher lot to lot specificity and tend towards more reproducible results. Since polyclonal antibodies contain a variety of clones and thus the potential to bind many different sites on the target protein they tend to have a strong signal and to work in a variety of applications.
The desired specificity of your secondary antibody depends on whether you are using poly- or monoclonal primary antibodies. Some secondary antibodies are broad and recognize multiple isotypes from a species, like a general anti-mouse antibody. Others recognize specific isotopes within that species, like an anti-mouse IgG antibody. Some are subclass specific, like an anti-mouse IgG2a antibody.
When using a monoclonal antibody be sure to use a highly specific secondary antibody, rather than a broadly reactive secondary (Fig. 1). For example, an anti-mouse IgG2a secondary would be a better choice for a mouse monoclonal IgG2a than a general anti-mouse IgG. In a broadly specific secondary antibody only a fraction of the antibody molecules will recognize and bind to your primary antibody. Manning et al. tested a variety of commercially available secondary antibodies and found that across suppliers there was a detection bias of IgG2a > IgG2b > IgG1 (Manning 2012).
-min.jpg?width=700&height=394&name=Isotype%20Considerations%20Draft%20Images%20(1)-min.jpg)
|
|
Figure 1: Broadly reactive versus subclass-specific antibodies. Only a fraction of the antibody molecules present in a broadly reactive secondary antibody will bind to a monoclonal primary antibody (A). To boost signal, consider using subclass specific secondary antibodies instead (B). |
Since a polyclonal primary antibody contains a mix of different isotypes and subclasses, use a broadly reactive secondary antibody to increase the chance of primary/secondary antibody binding and therefore the signal strength (Fig. 1).
Monoclonal primaries
If you do decide on a monoclonal antibody you may wonder, “Will the isotype, and subclass affect my experiment?” Well, it depends on the application and target. If you are running a basic western blot and probing a single target that expresses well, then probably not. In this case, take a look at the secondary antibodies that your lab has on hand and choose a primary antibody that will work with one of these. After all, there’s no sense in spending valuable research dollars on a new secondary antibody if you don’t need it.
If, on the other hand, your target is expressed at very low levels or your application requires a high degree of affinity, then species choice could very well matter. Take rabbit versus mouse monoclonal antibodies for example. Rabbit monoclonal antibodies have higher affinity and specificity than mouse monoclonal antibodies. Antibodies with higher affinities, like those from a rabbit, bind a greater amount of antigen in a shorter period of time and would likely perform better for probing poorly expressed targets. Similarly, rabbit derived antibodies may be the better choice for assays that require high affinity binding, such as immunoprecipitations.
Your sample species should also be considered when choosing an antibody. In some applications probing species-on-species, i.e. using a mouse derived primary antibody to stain a target protein in mouse tissues, causes a high degree of background staining. This occurs because the anti-mouse secondary antibodies used for detection bind to non-target immunoglobulins naturally present in the tissue sample. There are species-on-species staining protocols that use antibody fragments to block the endogenous immunoglobulins and reduce the background staining but they do not always eliminate the issue (Lu 1998). Consequently, if you find yourself in a sticky species-on-species situation, and other antibody options are available to you, it may be worth considering an antibody raised in a different species.
-min.jpg?width=889&height=500&name=Isotype%20Considerations%20Draft%20Images%20(2)-min.jpg)
|
| Table 1: Isotype considerations when choosing antibodies |
Complexity is a key factor
Biological systems tend to be complex with a variety of proteins interacting. Experiments tend to be equally complex with users trying to probe multiple targets in parallel. In these cases, it is critical that users choose antibodies that are easily distinguishable from each other. In a direct approach, that is simple - but what about when you need to use an indirect (primary and secondary antibody) approach?
Staining for multiple targets
When staining multiple targets indirectly, you must strategically plan primary/secondary antibody pairing such that each target has a unique species or isotype that can be probed with a distinctly conjugated secondary antibody. Consider too that some secondary antibodies can cross react with other species. For example, some goat anti-mouse secondary antibodies recognize some rat primary antibodies (Erickson 1993). It is always better to choose primary antibodies from more distantly related species and/or use narrowly specific secondary antibodies rather than generally reactive ones. And make sure to check for any reported cross-reactivity when planning your experiment.
When probing multiple targets in parallel, use the most narrowly specific secondary antibodies available to minimize the risk of cross-reactivity. To further limit species cross-reactivity, try to find secondary antibodies that are all derived from the same host species and have been cross-adsorbed. The cross-adsorption process filters out off-target antibodies increasing specificity and reducing cross-reactivity. For example, if you are using mouse IgG2a and rat IgG1 antibodies in the same application, using a Goat anti-mouse IgG2a cross-adsorbed secondary and a Goat anti-rat IgG1cross-adsorbed secondary would limit species cross-reactivity and therefore reduce the background staining (Table 2).
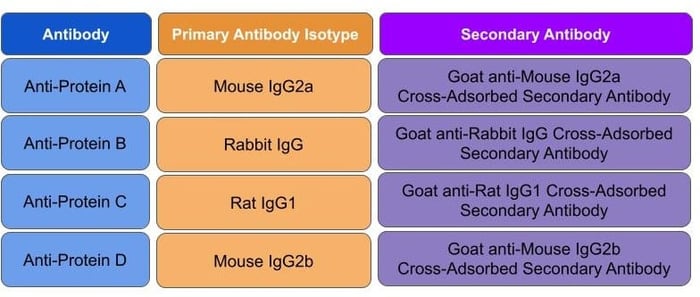
|
| Table 2: Secondary antibody selections for a four-antibody panel, staining in parallel |
In short, when staining multiple targets, look for primary antibodies raised in different, and distantly related, species, and secondary antibodies raised in the same species that have been cross-adsorbed to reduce off-target signals. Use isotype- or subtype-specific secondary antibodies when staining multiple targets with monoclonal primary antibodies, and use broadly reactive secondary antibodies when you’re using polyclonal primary antibodies.
I hope this post has explained when and why antibody isotypes matter and helps you choose the right antibody for your application!
References and resources
References
Lu, Q. L., & Partridge, T. A. (1998). A New Blocking Method for Application of Murine Monoclonal Antibody to Mouse Tissue Sections. In Journal of Histochemistry & Cytochemistry (Vol. 46, Issue 8, pp. 977–983). SAGE Publications. https://doi.org/10.1177/002215549804600813
Erickson, P. A., Lewis, G. P., & Fisher, S. K. (1993). Chapter 15 Postembedding Immunocytochemical Techniques for Light and Electron Microscopy. In Methods in Cell Biology (pp. 283–310). Elsevier. https://doi.org/10.1016/s0091-679x(08)60255-1
Manning, C. F., Bundros, A. M., & Trimmer, J. S. (2012). Benefits and Pitfalls of Secondary Antibodies: Why Choosing the Right Secondary Is of Primary Importance. In J. Sturtevant (Ed.), PLoS ONE (Vol. 7, Issue 6, p. e38313). Public Library of Science (PLoS). https://doi.org/10.1371/journal.pone.0038313
More resources from Addgene
Antibodies 101: Choosing the Right Antibody
Topics: Antibodies






Leave a Comment