Fighting with antibodies to produce immunohistochemistry images that are crisp, bright, and lacking in non-specific staining can be a challenge in the best of cases. But it can be particularly challenging when your only antibody option is from the same species as your tissue samples. The bad news: this situation is hard to avoid if your model system is a mouse, given the wealth of commercially available mouse monoclonal antibodies. The good news: there are strategies for mitigating this issue, and we’ve highlighted a few below.
What’s the problem?
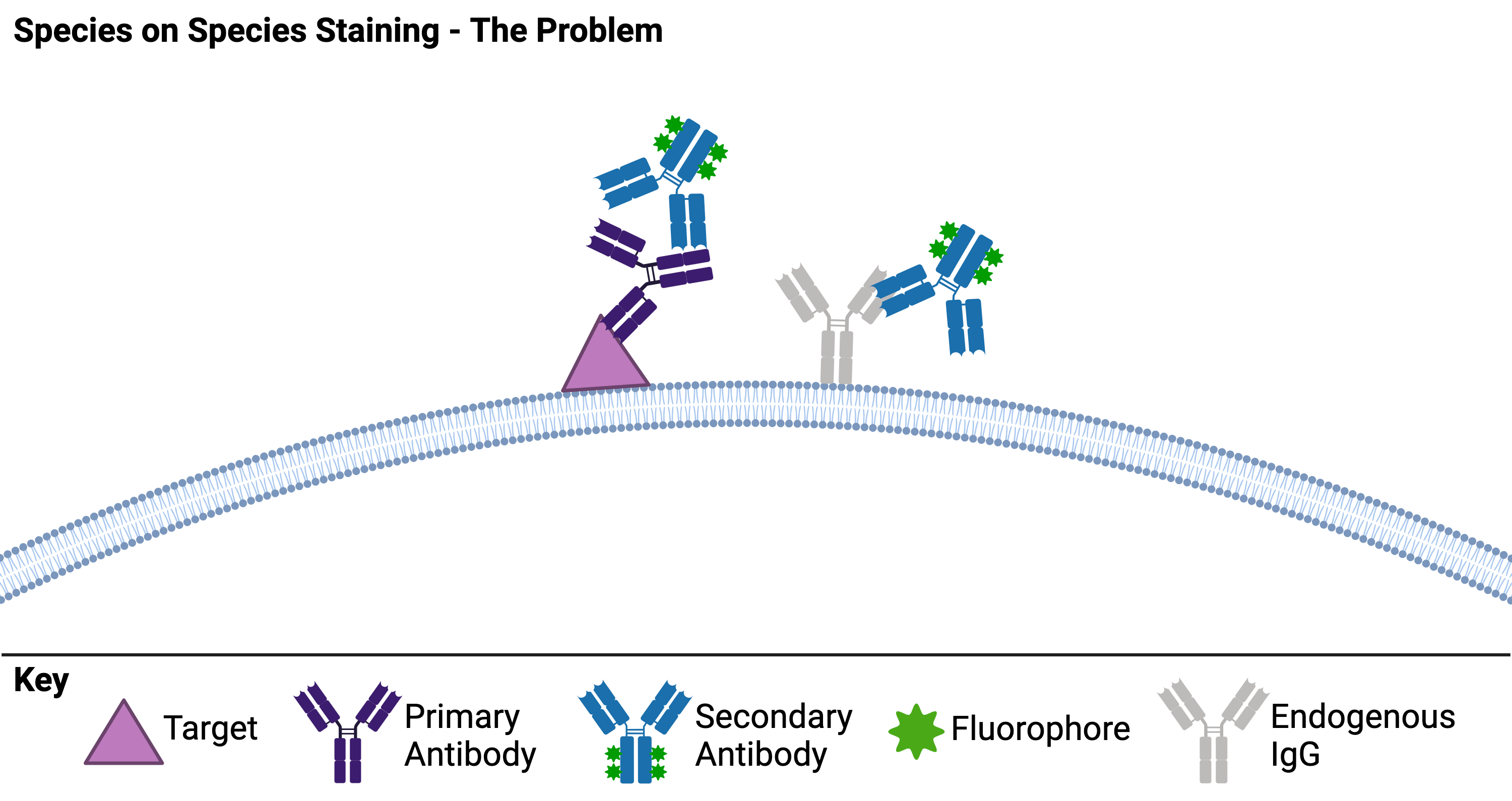
First, why are “mouse-on mouse” (or any “species on species”) immunochemistry (IHC) applications an issue? The culprits are endogenous IgGs in your tissue sample. Most common IHC protocols use two antibodies - one against your target of interest and a second against the first antibody. When endogenous IgGs of the same species as your primary antibody are present, your secondary antibody recognizes both the endogenous IgGs and your primary antibody, resulting in significant background staining (Figure 1A). Not all tissues will have the same levels of endogenous IgGs, so some researchers can get away with species on species staining with no problem! But to be sure that your secondary is not binding endogenous IgGs, you should include no primary antibody controls in your experiments. If you do find high levels of background staining by the secondary, then the following three strategies might help!
|
| Figure 1: Species on species staining can be a problem because the secondary antibody is not able to distinguish between the primary antibody and endogenous IgGs in the tissue. Created with biorender.com. |
Strategies for avoiding background secondary binding
Direct immunohistochemistry (Figure 2A)
How do you avoid having your secondary antibody bind endogenous IgGs? Don’t use one. If it is possible, using a primary antibody that is directly conjugated to your reporter circumvents the issue. However, directly conjugated primary antibodies can be expensive and may not provide enough signal to visualize low-abundance targets.
Pre-formed primary and secondary antibody complexes (Figure 2B)
Some labs have found that you can significantly decrease background staining by mixing your primary and secondary antibodies, blocking excess secondary with serum from the same species as your primary/sample, and then applying this mixture to your tissue (Tuson, et al., 1990; Goodpaster, et al., 2013). One issue with this strategy is that large complexes may not permeate through your tissue samples as homogeneously as a single antibody. Using a secondary antibody that is a Fab fragment, single-chain variable fragment (scFv), or a nanobody is one potential remedy for this problem within a problem.
Pre-block with Fab fragments (Figure 2C)
Speaking of Fab fragments… Another strategy for species-on-species IHC protocols is to block endogenous IgGs by pre-treating the tissue with unconjugated Fab fragments that recognize the endogenous IgGs (Lu and Partridge, 1998). After washing away unbound fragments, you can then apply your primary and secondary antibodies as normal. Ideally, your secondary will only bind to your primary in this scenario because the endogenous targets will have already been bound up by the Fab fragments. Note that you should plan to use Fab fragments that are from the same host species as your secondary antibody since 1) your secondary should not recognize Fab fragments from the same species and 2) these fragments will be more likely to bind (and block) the same epitopes recognized by the secondary.
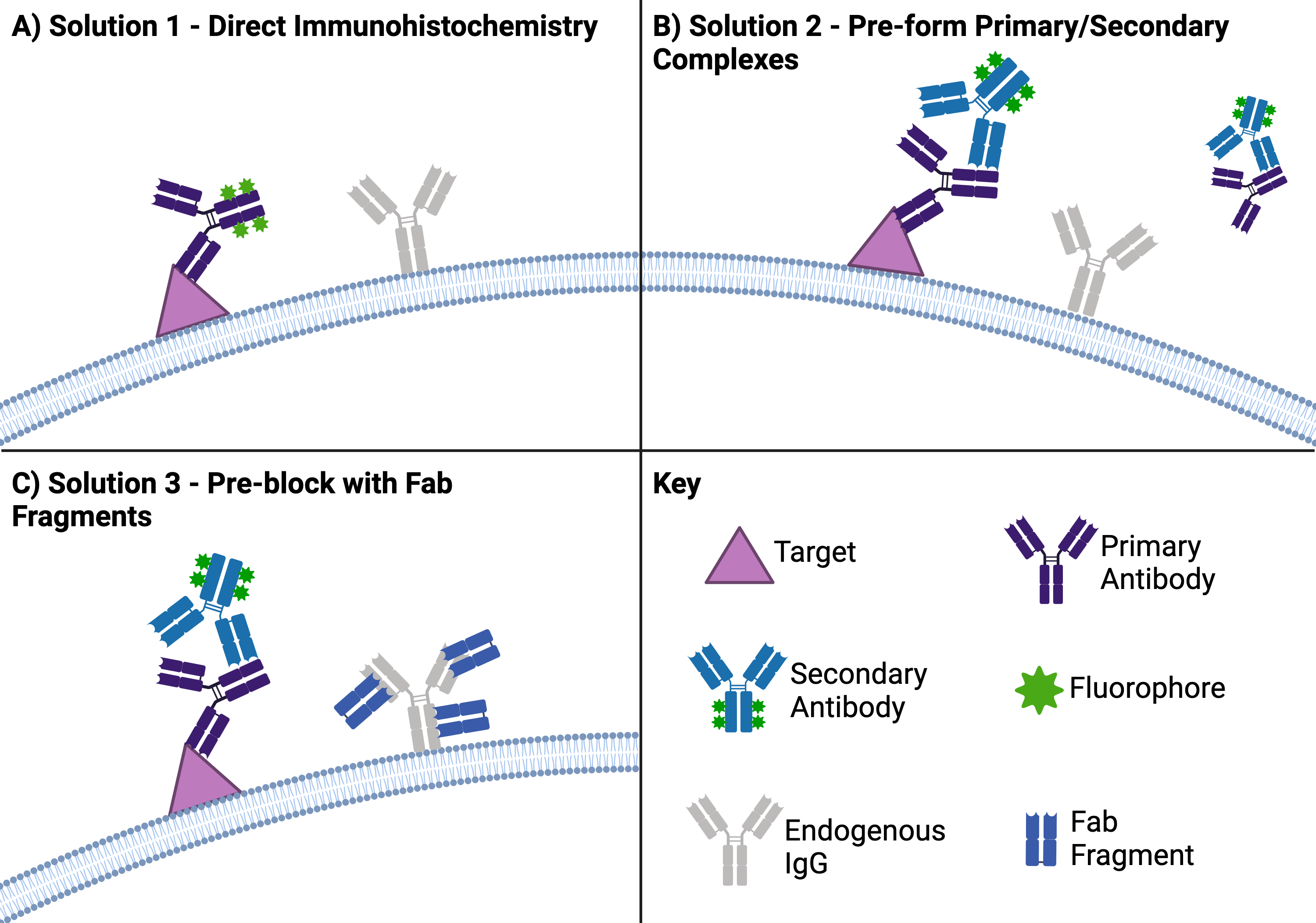 |
| Figure 2: There are a few common solutions to avoid background staining when doing species on species IHC. (A) One solution is to use direct IHC, which uses just a primary antibody that has your reporter (in this case a fluorophore) directly attached to it. (B) Another solution is to combine your primary and secondary antibodies before adding them to your sample so the secondary antibodies aren’t free to bind endogenous IgGs. (C) A third solution is to use Fab fragments against the sample species to block the binding sites on endogenous IgGs before you add your primary and secondary antibodies. Created with biorender.com. |
Proper tissue processing and sample preparation can also help reduce the levels of endogenous IgGs in your samples, but know that all is not lost if you still see non-specific staining! Hopefully, these solutions will help get your experiments back on track.
One final note — this post focuses on the species-on-species problem in the context of IHC, but the problem can impact other techniques like Western blots or immunocytochemistry (ICC). Not all of the solutions described here may be as effective for other techniques, but it is important to be aware that the issue is not unique to IHC.
References and Resources
References
Goodpaster T, Randolph-Habecker J (2013). A Flexible Mouse-On-Mouse Immunohistochemical Staining Technique Adaptable to Biotin-Free Reagents, Immunofluorescence, and Multiple Antibody Staining. J Histochem Cytochem 62:197–204. https://doi.org/10.1369/0022155413511620
Lu QL, Partridge TA (1998). A New Blocking Method for Application of Murine Monoclonal Antibody to Mouse Tissue Sections. J Histochem Cytochem 46:977–983. https://doi.org/10.1177/002215549804600813
Tuson JR, Pascoe EW, Jacob DA (1990) A novel immunohistochemical technique for demonstration of specific binding of human monoclonal antibodies to human cryostat tissue sections. J Histochem Cytochem : Off J Histochem Soc 38:923–926. https://doi.org/10.1177/38.7.2355173
Additional resources on the Addgene blog
- Antibodies 101: Introduction to Immunofluorescence
- Antibodies 101: Affinity Reagents
- Antibodies 101: Single-Chain Variable Fragment (scFvs)
Resources on Addgene.org
- Recombinant antibodies for IHC at Addgene
Topics: Antibodies





Leave a Comment