This post was contributed by guest blogger Harshana S De Silva Feelixge.
Gene therapy technologies hold great promise for improving or potentially curing human diseases that were previously thought to be incurable. Rapid advances in next generation sequencing technologies have allowed scientists to quickly identify underlying genetic causes of some human conditions, opening up new avenues for therapeutics that treat disease at the molecular level. For instance, if a disease is caused by a mutation in a single gene, it can potentially be treated by correcting the mutation or replacing the gene. A notable example is the treatment of Severe Combined Immune Deficiency disease (SCID-XI), also known as bubble boy syndrome. This disease is caused by mutations in the common cytokine receptor gamma chain (c) and is characterized by a lack of immune cell development and function. To date, gene therapy has been used to treat 10 infants with this disease. To do so, their T-cells were grown in vitro, their mutations corrected, and the T-cells were transferred back into the infants. Almost all patients have achieved persistent immunological reconstitution with a normally functioning T cell repertoire (1).
In this example, it was possible to transduce the T-cells outside the body and thereby correct the mutations using retroviral vectors. However, many diseases require techniques that can deliver gene therapies directly to the cells that need them. Adeno-associated virus (AAV) – based delivery techniques may hold the key.
In the simplest form, translating gene therapies from conceptual design to clinical trials involve identifying a therapeutic gene, finding a means to deliver it and identifying a suitable route of administration. However, until recently safe delivery of nucleic acid cargo (DNA or RNA) to target cells has been challenging. A variety of vector delivery technologies have been investigated including lentiviruses, adenovirus, and inorganic delivery tools such as nanoparticles. These technologies can have serious adverse effects, and their immunogenic or carcinogenic profiles have limited their usability in clinical settings. On the other hand, vectors derived from AAV have recently gained popularity as they are uniquely suited gene delivery vehicles with great safety profiles as well as other benefits.
What makes AAV vectors great gene delivery tools?
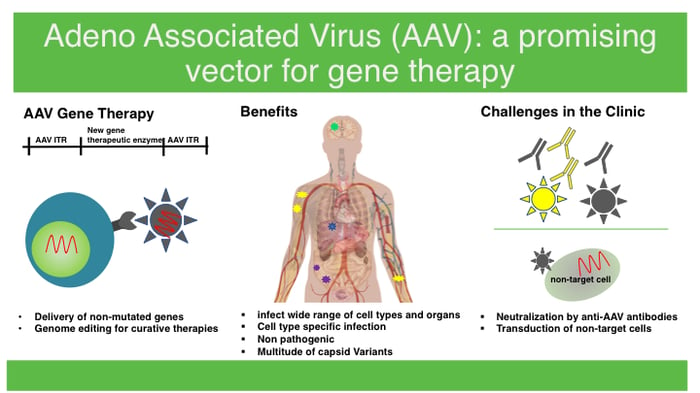
There are a variety of key properties that distinguish AAVs as gene delivery tools:
- Unlike most naturally occurring viruses used in clinical trials, wild type AAV is not associated with any pathogenicity and has a low immunogenic profile. AAV was first discovered as a contaminant of adenovirus preparations. It is an icosahedral, non-enveloped virus that carries a small, approximately 4.7 kb single stranded genome. AAV belongs to the family Parvoviridae and requires co-infection of helper viruses like adenovirus for productive infection. AAV vectors are engineered to provide additional safety benefits: they lack all viral genes, including those that are responsible for integration into host chromosomes, further minimizing potential activation of innate immunity.
- AAV vectors are also capable of infecting a wide range of cell types including but not limited to muscle, liver, and brain cells. There are a multitude of known AAV serotypes that have slight variations in their viral capsids. Novel forms of AAV capsids are discovered frequently and recent advances in AAV technology have enabled us to generate new and refined forms of capsids with improved specificity.
- AAV vectors are able to facilitate and enhance DNA repair via homology directed repair (HDR). This characteristic makes AAV vectors especially amenable to correcting disease-associated mutations.
- AAV vectors maintain persistent transgene expression over many years in postmitotic, long-lived cell types.
AAVs in the clinic
Thus far, AAV vectors AAV1, AAV-2, AAV1-AAV2 hybrids, AAV-6, AAV-7, AAV-8, AAV-9 and AAVrh10 have been used in more than 183 gene therapy trials (http://www.abedia.com/wiley/vectors.php). These clinical trials have been directed at diseases including but not limited to hemophilia B, LPL deficiency, Cystic fibrosis, Muscular Dystrophy, Parkinson’s disease, and HIV. A notable landmark study showed significant clinical efficacy against a form of blindness known as Leber’s congenital amurosis (LCA). This disease is caused by mutations in the gene RPE65. It is characterized by childhood onset of blindness and was thought to be untreatable until 2008. Following a single dose of subretinal administration of rAAV2 carrying the human gene RPE65, patients with clinical diagnosis of LCA and mutations in RPE65 (n=3), showed significant improvements in vision over a period of 1 to 3 years – the treatment appears to be both safe and effective.
Challenges for AAV gene therapy
Although AAV-based gene therapies are promising, scientists are working to overcome key challenges to their efficacy:
- Pre-existing immunity: Many individuals already have wt AAV in their bodies and may therefore already have ways to prevent AAV infection. For example, prevalence of neutralizing antibodies against some AAV types, AAV1, 2, 3 and 5 are thought to be as high as 70% among the human population. These pre-existing antibodies can interfere with virus-cell interactions and seriously hinder gene therapy outcomes.
- Anti-AAV antibodies developed by the immune system – patients’ own immune systems can develop antibodies that neutralize the AAV vectors used to treat them. Scientists are working to overcome this issue by developing new capsids through direct evolution, capsid shuffling, and peptide displays. These capsids will be selected both for their ability to evade the immune system and for enhanced abilities to infect target tissues. Improved specificity through control of gene expression and the selection of specific promoters may also help AAV vectors evade host immunity.
While basic research and other ongoing clinical trials support the utility of AAV as a broadly applicable gene delivery tool, aspects of AAV biology should be taken into account when developing AAV-based therapies. Furthermore, deeper understanding of disease pathogenesis will enable robust therapeutic outcomes. In contrast to other gene delivery tools that are under consideration, recent advancements in various AAV technologies have vastly improved AAVs’ potential to become ideal vectors for gene delivery.
Many thanks to our guest blogger, Harshana S De Silva Feelixge.
 Harshana S De Silva Feelixge is a researcher whose work has focused on gene therapy for viral infections. She is particularly passionate about HIV cure research and science communication.
Harshana S De Silva Feelixge is a researcher whose work has focused on gene therapy for viral infections. She is particularly passionate about HIV cure research and science communication.
References
1. Gaspar, H. B., S. Howe, and A. J. Thrasher. "Gene therapy progress and prospects: gene therapy for severe combined immunodeficiency." Gene therapy 10.24 (2003): 1999-2004. PubMed PMID: 14566358.
2. Hirsch, M. L., et al. "Self-complementary AAV mediates gene targeting and enhances endonuclease delivery for double-strand break repair." Gene therapy 17.9 (2010): 1175-1180. PubMed PMID: 20463753. PubMed Central PMCID: PMC3152950.
3. Jacobson, Samuel G., et al. "Improvement and decline in vision with gene therapy in childhood blindness." New England Journal of Medicine 372.20 (2015): 1920-1926. PubMed PMID: 25936984. PubMed Central PMCID: PMC4450362.
4. Sun, J. Y., et al. "Immune responses to adeno-associated virus and its recombinant vectors." Gene therapy 10.11 (2003): 964-976. PubMed PMID: 12756417.
5. Chan, Ken Y., et al. "Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems." Nature Neuroscience 20.8 (2017): 1172-1179. PubMed PMID: 28671695.
6. Münch, Robert C., et al. "Off-target-free gene delivery by affinity-purified receptor-targeted viral vectors." Nature communications 6 (2015): ncomms7246. PubMed PMID: 25665714.
Additional Resources on the Addgene Blog
- Check out our Viral Vectors Featured Topic Page
- Learn about CRISPR-AAV vectors
- Learn about Addgene AAV Quality Control Process
Resources on Addgene.org
- Find ready-to-use AAV in Addgene's Viral Service
- Check out our AAV Production Protocol
- Check out our AAV Purification Protocol
Topics: Viral Vectors, AAV








Leave a Comment