Viruses have many negative associations: common colds, COVID-19, norovirus (the stomach flu), and many more. Their infectious nature allows them to easily deliver their “cargo” to target cells and organisms, and when that cargo is designed to make you sick…well, you feel it. But the same features that make these familiar viruses such a pain is exactly why viruses are such wonderful scientific and medical tools. As scientists, we can manipulate what this cargo is and where it goes, turning these pesky germs into useful tools. Here, we will review viral basics and the types of viruses used as biological tools.
Viruses as biological tools
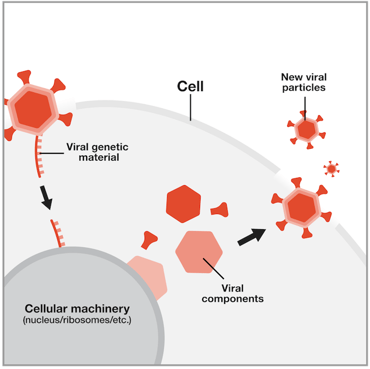
In nature, viruses infect cells and organisms (which we refer to as hosts) via viral particles, the functional infection unit. Each particle has a viral envelope, which fuses with the target cell’s membrane, allowing the viral particle access into the cell. The viral envelope and its proteins determine what type of cell the virus will target and fuse with. In nature, this determines which organisms and cells they infect. In the lab, this means viruses can be engineered to be cell type specific. Once inside the host cell, the virus unleashes its cargo – its own genome.
|
| Fig. 1: Viral particle infection into a host cell. Image courtesy of National Human Genome Research Institute. |
Its genome, however, isn’t enough to ensure the virus can replicate and spread. Instead, viruses replicate by hijacking the host’s system to produce more virus and continue the infection cycle. Depending on the type of virus used, the DNA (or RNA) cargo will either permanently integrate into the host genome or temporarily express whatever it encodes. This ability to infect cells with a high efficiency and deposit a DNA/RNA cargo into their targets makes them attractive and tractable tools for genome engineering. Scientists have engineered viruses to target specific cell types and deliver any cargo you fancy (size limits do apply, however.)
Why use a virus?
Viruses are essentially DNA (or RNA) delivery vectors, so what makes them better than the alternatives? Well, for one they have a high transduction efficiency and can effectively infect a wide range of cell types that are typically ‘difficult to transfect’, such as primary cells. They can also be directly injected into hard-to-reach organs, such as the brain. Secondly, some viruses have a low immunogenicity which makes them ideal for applications with whole organisms. Finally, viral delivery is typically straightforward across applications, while electroporation of a human, for example, isn’t a reasonable option.
Since viruses used in labs are derived from viruses that infect people in nature, extra safety measures, like replication incompetent viral particles, are required. We will be covering these safety measures in a separate, upcoming post.
If a viral vector seems like the right approach for your experiment, you’ll need to decide exactly which type of virus to use.
Types of viruses
There are four main types of viruses used in mammalian research: 𝛄-retrovirus, lentivirus, adenovirus, and adeno-associated virus (AAV). While each virus allows you to infect cells and deliver cargo, it’s important to understand their differences to choose the best one for your experiment.
Retro and lenti viruses
Retroviruses are a family of viruses that stably integrate their genome into the host genome, setting up permanent residence. They primarily infect dividing cells, as their access to the host genome requires breakdown of the nuclear envelope, which occurs during mitosis. The most well-known retrovirus is HIV.
Lentiviruses are a genus of the retroviral family that can infect both non-dividing and dividing cells. They accomplish this through nuclear localization signals which can traffic their viral particles inside the nucleus, without the need for envelope disassembly. Both lenti and retro viruses can accommodate a cargo up to approximately 8 kb, but viral packaging is less efficient when inserts are on the higher end.
Adeno and adeno-associated viruses
Adenoviruses infect non-dividing cells but they do not permanently integrate into the host genome (non-integrating) as lenti and retro viruses do. They are generally used to achieve transient, high expression of the relevant cargo without the fear of random genomic integration causing mutagenesis in the host. Adenoviruses can deliver relatively large cargo inserts (>8 kb).
Adeno-associated viruses (AAVs) are also non-integrating and infect non-dividing cells. AAVs primary advantage over adenovirus is their low immunogenicity, which means they do not stimulate a strong immune response when introduced into an organism. This feature makes them an attractive tool for human subject delivery, as immunogenicity was previously a large barrier for gene therapy. In fact, AAV gene therapies have already been approved in the US for multiple diseases, including hemophilia B and spinal muscular atrophy.
| Type of Virus | Pros | Cons |
|
Retrovirus
|
|
|
|
Lentivirus
|
|
|
|
Adenovirus
|
|
|
|
AAV
|
|
|
From basic science to clinical applications, viruses are true bench to bedside tools. Keep an eye out for our upcoming blogs on virus safety and viral applications to help you start (safely!) thinking about the possibilities.
Ready to plan your next (or maybe first!) viral experiment?
Click Here to Find Viral Vector Resources at Addgene!
References and Resources
1. Roe T, et al. “Integration of murine leukemia virus DNA depends on mitosis.” EMBO J. 12 (1993) 2099-2108. PubMed PMID: 8491198. PubMed Central PMCID: PMC413431.
2. Bukrinsky MI, et al. “Active nuclear import of human immunodeficiency virus type 1 preintegration complexes.” Proc. Natl. Acad. Sci. U.S.A. 89 (1992) 6580–6584. PubMed PMID: 1631159. PubMed Central PMCID: PMC49545.
3. Coffin J, Hughes S, Varmus H. Retroviruses. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 1997. PubMed PMID: 21433340.
4. Cronin J, et al. “Altering the tropism of lentiviral vectors through pseudotyping.” Curr. Gene. Ther. 5 (2005) 387-398. PubMed PMID: 16101513. PubMed Central PMCID: PMC1368960.
Additional Resources on the Addgene Blog
- Learn More about Lentivirus
- Listen to Connie Cepko Discuss Gene Therapy with AAV
- Figure out How to Get the Most from Your Lentiviral Transduction
Additional Resources on Addgene.org
Topics: Viral Vectors, Viral Vectors 101










Leave a Comment