To deliver genes using lentiviral vectors, you need an envelope protein on the virus’s surface and a corresponding receptor in the host cell. Some of these envelope-receptor pairings are broad, allowing delivery into many cell types, while others are specific, allowing delivery to only a few cell types.
If you’re looking for more control in the cell types your lentiviral vector will infect, pseudotyping can help. In pseudotyping, you produce viral vectors using viral envelope proteins from another virus to either restrict or broaden the host cell range (tropism). Pseudotyping is only done on enveloped viruses such as lentivirus, retrovirus, and rabies virus.
How to pseudotype
To pseudotype your viral vector, you use the same protocol for generating lentivirus, but you use a different envelope glycoprotein instead of the wild-type glycoprotein. The pseudotyped protein is encoded on the envelope plasmid which contains a promoter, the envelope gene, and a polyA tail.
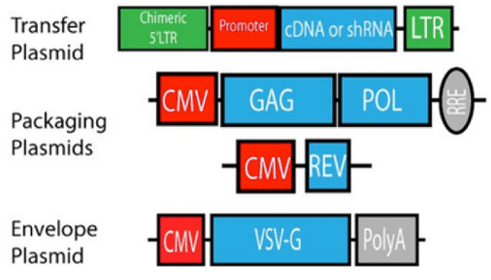 |
| Figure 1: Lentiviral production uses three plasmids: (1) The transfer plasmid, (2) the envelope plasmid, and (3) the packaging plasmid. These are transfected into target cells which produce lentiviral particles. |
Why pseudotype?
Lentiviruses and retroviruses typically target immune cells with their wild-type envelopes. However, pseudotyping allows these viruses to infect neurons. Besides altering the host tropism of your virus, there are many other reasons when you might pseudotype your virus:
- Lowering cytotoxicity. Different envelope proteins have different levels of cytotoxicity and the mechanisms behind the cytotoxicity can vary. For example, the popular VSV-G envelope protein causes cells that it infects to fuse together and form syncytias that cause the cells to die. Cytotoxicity can also be cell type dependent: if a cell has few receptors for a specific envelope protein, it takes up less of the virus and is less affected.
- Altering sensitivity to serum. Different pseudotyped viruses are differentially inactivated by nonspecific complement mechanisms in serum. Sometimes this is due to the cells that the viral vectors were produced in. For example, production of vesicular stomatitis virus, HIV-2, and human foamy virus in cell lines expressing galactosyl(alpha1-3)galactosyl (alphaGal) sugars were less stable than viruses produced in cell lines that do not express these sugars. (Takeuchi et al., 1997). The alphaGal sugars end up in the envelope and are targets for complement-based killing by anti-alphaGal antibodies. The VSV-G envelope protein is inactivated by serum regardless of the producer cell type, but other envelopes such as the one from gibbon ape leukemia virus (GALV) are more stable (Sandrin et al., 2002).
- Studying a viral pathogen more safely. Recently, many SARS-CoV-2 researchers began pseudotyping the SARS-CoV-2 spike protein into a lower BSL virus like lentivirus (Crawford et al., 2020). That way, they can study the spike protein’s role in infecting cell lines without using intact SARS-CoV-2 virus. Scientists have been using similar pseudotyping methods with other viruses in a BSL-2 lab instead of a BSL-3 or BSL-4 lab. For more details, check out this review article published in Reviews in Medical Virology (Li et a., 2018).
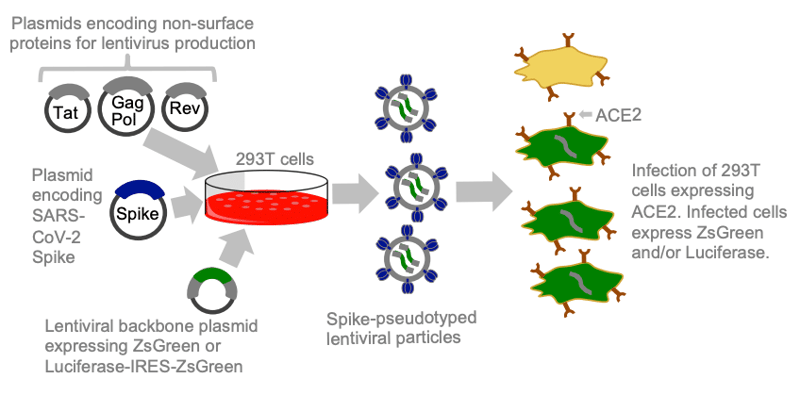 |
| Figure 2: To pseudotype with the SARS-CoV-2 spike protein, scientists encode the spike protein on the envelope plasmid. Image from Crawford et al., 2020. |
Broad tropism: VSV-G pseudotyping
A commonly used envelope protein for pseudotyping is VSV-G, or vesicular stomatitis virus G protein. VSV-G is a trimeric protein that binds phosphatidylserine and low-density lipoprotein receptors (LDLRs) on a cell surface to endocytose into the cell.
The reason VSV-G is so commonly used is that it is good at infecting most cells, but not all. For example, LDLR expression in hematopoietic stem cells, T cells, and B cells is low. Infection of these cells with VSV-G pseudotyped virus requires the upregulation of LDLR expression using other methods.
In terms of preparation, VSV-G is stable and can withstand ultracentrifugation which helps scientists achieve a high titer (reviewed in Joglekar and Sandoval, 2017). This high titer is one of the reasons VSV-G is used to transduce stem cells and neurons.
However, VSV-G’s broad tropism can be undesired for in vivo studies since there’s the potential for off-target infectivity as it can bind and transduce many cell types before reaching the target cells. VSV-G is also toxic at high concentrations as described above and is inactivated by the complement system in serum (DePolo et al., 2000).
Other envelope proteins for pseudotyping
While VSV-G is the most commonly used envelope protein for pseudotyping, there are many more envelope proteins that can be used for specific purposes as described previously (Table 1, Gutierrez-Guerrero, et al, 2020). Because retrovirus is closely related to lentivirus, many retroviral envelope proteins have been pseudotyped on lentiviral particles.
Rabies glycoprotein
The rabies glycoprotein targets neuronal cells and can travel in a retrograde direction from the synapse to the cell body, making it a great tool to study neuronal connectivity. However, because of rabies virus’s toxicity, it made more sense to pseudotype another virus with rabies glycoprotein. Lentiviral vectors are also great at infecting neurons and cause minimal inflammatory response, but are not taken up by axon terminals and can’t be used for neuronal tracing or peripheral gene delivery. Combining the rabies glycoprotein into the lentiviral envelope allows the lentiviral vector to be transported retrograde and also has higher neuronal transduction efficiency and a better safety profile than either virus exhibits alone.
This pseudotyped lentivirus with rabies glycoprotein now serves as a retrograde tracer so that scientists can identify the neurons that project to a given brain region (Mazarakis et al., 2001). This method can also allow scientists to trace a neuron’s projections back to the cell body.
Rabies virus and the EnvA-TVA system
Another way to use rabies virus is to delete the glycoprotein required for entry into the cell (rabies dG). Because rabies dG can’t infect neurons on its own, it needs to be pseudotyped. This takes advantage of the EnvA-TVA receptor-envelope system (Wickersham et al., 2007). This system requires first supplying rabies glycoprotein along with the TVA receptor in neurons using AAV and then delivering rabies dG pseudotyped with EnvA. This allows rabies dG to only infect neurons that express TVA and the rabies glycoprotein. The glycoprotein will be incorporated into the replicating rabies virus envelope, travel retrogradely, and jump the synapse to the previous neuron (Wichersham et al., 2007). However, once rabies dG enters this neuron, it cannot infect any subsequent neurons because it does not express rabies glycoprotein.
Other pseudotyping options
There are many, many other options for envelope proteins for pseudotyping that could be broad or specific to suit your needs. Check out some of these review articles to find tables and information on different viral membranes, tropism, and advantages and disadvantages:
- Table 1 (Joglekar et al., 2017)
- Table 1 (Gutierrez-Guerrero et al., 2020)
- Table 1 (Cronin et al., 2005)
References and Resources
References:
Crawford KHD, Eguia R, Dingens AS, Loes AN, Malone KD, Wolf CR, Chu HY, Tortorici MA, Veesler D, Murphy M, Pettie D, King NP, Balazs AB, Bloom JD (2020) Protocol and Reagents for Pseudotyping Lentiviral Particles with SARS-CoV-2 Spike Protein for Neutralization Assays. Viruses 12:513 . https://doi.org/10.3390/v12050513
Cronin J, Zhang X-Y, Reiser J (2005) Altering the Tropism of Lentiviral Vectors through Pseudotyping. CGT 5:387–398 . https://doi.org/10.2174/1566523054546224
DePolo NJ, Reed JD, Sheridan PL, Townsend K, Sauter SL, Jolly DJ, Dubensky TW Jr (2000) VSV-G Pseudotyped Lentiviral Vector Particles Produced in Human Cells Are Inactivated by Human Serum. Molecular Therapy 2:218–222 . https://doi.org/10.1006/mthe.2000.0116
Gutierrez-Guerrero A, Cosset F-L, Verhoeyen E (2020) Lentiviral Vector Pseudotypes: Precious Tools to Improve Gene Modification of Hematopoietic Cells for Research and Gene Therapy. Viruses 12:1016 . https://doi.org/10.3390/v12091016
Joglekar AV, Sandoval S (2017) Pseudotyped Lentiviral Vectors: One Vector, Many Guises. Human Gene Therapy Methods 28:291–301 . https://doi.org/10.1089/hgtb.2017.084
Li Q, Liu Q, Huang W, Li X, Wang Y (2017) Current status on the development of pseudoviruses for enveloped viruses. Rev Med Virol 28:e1963 . https://doi.org/10.1002/rmv.1963
Mazarakis ND (2001) Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Human Molecular Genetics 10:2109–2121 . https://doi.org/10.1093/hmg/10.19.2109
Sandrin V, Boson B, Salmon P, Gay W, Nègre D, Le Grand R, Trono D, Cosset F-L (2002) Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood 100:823–832 . https://doi.org/10.1182/blood-2001-11-0042
Takeuchi Y, Liong SH, Bieniasz PD, Jäger U, Porter CD, Friedman T, McClure MO, Weiss RA (1997) Sensitization of rhabdo-, lenti-, and spumaviruses to human serum by galactosyl(alpha1-3)galactosylation. Journal of virology 71:6174–6178 . https://doi.org/10.1128/jvi.71.8.6174-6178.1997
Wickersham IR, Finke S, Conzelmann K-K, Callaway EM (2006) Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods 4:47–49 . https://doi.org/10.1038/nmeth999
Wickersham IR, Lyon DC, Barnard RJO, Mori T, Finke S, Conzelmann K-K, Young JAT, Callaway EM (2007) Monosynaptic Restriction of Transsynaptic Tracing from Single, Genetically Targeted Neurons. Neuron 53:639–647 . https://doi.org/10.1016/j.neuron.2007.01.033
Additional resources on the Addgene blog:
Resources on Addgene.org:
- Check out the lentivirus guide
- Find ready-to-use lentiviral preps at Addgene
Topics: Viral Vectors, Viral Vectors 101, Retroviral and Lentiviral Vectors







Leave a Comment