So you have this awesome experiment you want to do, but it requires some AAV. You’ve never worked with AAV before, but you aren’t going to let that stop you. Where do you start? Turns out like all good experiments, making AAV starts with some plasmids. You just need three plasmids to start making AAV:
- the packaging plasmid which contains the structural and packaging genes,
- the adenoviral helper plasmid which contains the proteins needed for the virus to replicate,
- and the transfer plasmid which contains the viral genome.
In today’s blog post, we’ll focus on the AAV transfer plasmid and take a look at each of its parts.
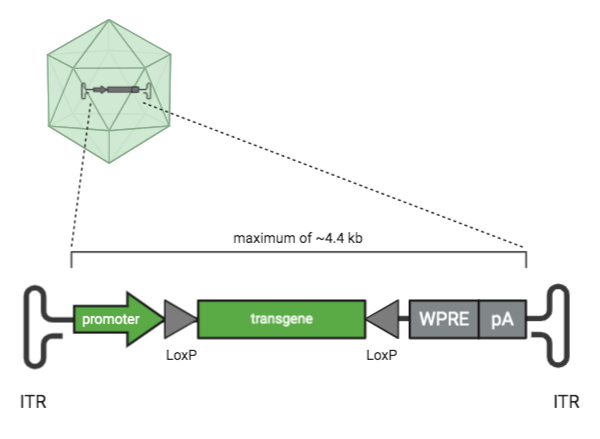 |
| Figure 1: Parts of an AAV transfer plasmid. Parts shown in green are key elements of an AAV plasmid. Parts shown in grey are optional cis-regulatory elements. |
Key elements of an AAV plasmid
First let’s talk about the key parts of an AAV plasmid.
Inverted terminal repeat (ITR) sequence
Inverted terminal repeats (ITRs) are what makes an AAV transfer plasmid an AAV transfer plasmid. ITR sequences are 145 bases each and AAV plasmids have two ITR sequences. The DNA sequence between the ITRs is what gets packaged into the AAV molecule. What’s outside of the ITRs does not get packaged in the AAV vector. The cloning capacity between the ITRs is ~4.4 kb.
ITRs are inherently unstable due to their secondary structure, palindromic nature, and high GC content. This instability can cause partial loss of an ITR during routine plasmid propagation, with complete ITR loss abolishing AAV packaging. To prevent ITR loss, it’s best to propagate AAV constructs in recombination-deficient bacterial strains, such as Stbl3s. ITR loss can be detected by restriction digest using enzymes, like SmaI, which have recognition sites in the ITRs. Regular Sanger sequencing typically fails to sequence through ITRs due to their secondary structure and high GC content, but some companies now offer Sanger sequencing conditions which can read through this challenging sequence.
Transgene
The transgene is the gene that will be delivered by the AAV. On a plasmid map, its sequence is located between the ITRs. It can be anything you like as long as it’s ~4.4 kb or smaller. Some examples include a reporter like GFP, some smaller CRISPR-Cas proteins such as SaCas9, CRISPR gRNAs, or your gene of interest.
Promoter
The promoter drives the expression of the AAV transgene. On a plasmid map, promoters are upstream or 5’ of the gene they control. Promoters can be organism or tissue type-specific, which can help you restrict the expression of your transgene to a specific species or location. Promoters can also drive different levels of expression. For example, CAG is a strong promoter that drives very high, ubiquitous expression, while the human synapsin 1 promoter is neuronal specific (Haery et al., 2019). Most AAV transfer plasmids have a promoter, but this element isn’t required when the transfer plasmid harbors non-coding sequences.
Other cis-regulatory elements
Next let’s talk about some of the elements that aren’t required parts of an AAV transfer plasmid, but are often seen in them.
Lox sites
Lox sites, in combination with the Cre recombinase, are used to spatially and temporally control transgene expression. Lox sites are directional 34 bp sequences that can flank the transgene and are located between the ITRs. Transfer plasmids often have two pairs of lox sites which are part of a dual lox system called FLEX or DIO.
Lox sites are recognized by the recombinase Cre. Cre first flips the sequence between the first pair of lox sites, and then excise the sequence between the second pair. The final result is a permanently flipped transgene. By restricting the availability of Cre, lox sites lets researchers control the spatial and temporal activation of a transgene.
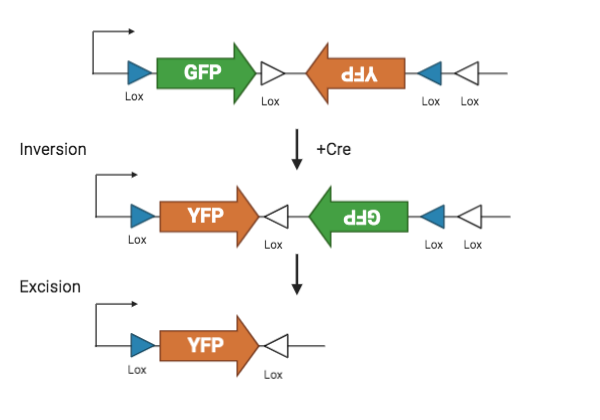 |
| Figure 2: FLEX Cre-Lox system. |
WPRE
The Woodchuck Hepatitis Virus (WHP) Posttranscriptional Regulatory Element (WPRE) forms a tertiary structure when transcribed which aids in nuclear export of mRNA and helps increase the expression of your transgene. The WPRE is located at the end of the AAV genome, just upstream of the final ITR.
polyA
The polyadenylation or polyA signal aids in the nuclear export of RNA and RNA translation, and promotes RNA transcript longevity. The polyA is located at the end of the AAV genome, just upstream of the final ITR and downstream of the WPRE.
TL;DR? Watch our AAV Transfer Plasmid video!
Now you’ve got a basic understanding of some common AAV plasmid elements! And while there are many other AAV plasmid elements and combinations out there not discussed in this post, you’ve got the knowledge to be able to design a construct for the successful and specific expression of your gene of interest.
References
Haery L, Deverman BE, Matho KS, Cetin A, Woodard K, Cepko C, Guerin KI, Rego MA, Ersing I, Bachle SM, Kamens J, Fan M (2019) Adeno-Associated Virus Technologies and Methods for Targeted Neuronal Manipulation. Front Neuroanat 13: . https://doi.org/10.3389/fnana.2019.00093
Wilmott P, Lisowski L, Alexander IE, Logan GJ (2019) A User’s Guide to the Inverted Terminal Repeats of Adeno-Associated Virus. Human Gene Therapy Methods 30:206–213 . https://doi.org/10.1089/hgtb.2019.276
Additional resources on the Addgene blog
- Learn more about basic viral vector elements
- Read about four considerations for the perfect AAV tool
- Learn about synthetic promoter AAVs for cell-type expression in retinal cells
Resources on Addgene.org
- Find AAVs for your experiment
- Browse our AAV protocols
Topics: Viral Vectors, Viral Vectors 101, AAV







Leave a Comment