Adeno-associated virus (AAV) is a popular tool for gene delivery, but it has a drawback: how do you ensure your gene goes where you want it to? Knowing that a gene is expressed in a particular cell type is important not only for translational research, such as gene therapy, but also basic research. To improve cell-type specificity of AAV, work has focused on modifying the outside protein shell, or capsid, of the virus so that it only enters and delivers it cargo to certain cell types.
Botond Roska’s lab at the Institute of Molecular and Clinical Ophthalmology Basel recently took a different approach to enhance specificity in retinal cells. To do this, the group developed a new strategy for finding retinal cell-type specific promoters. Most existing promoters express in a broad number of cell types, such as inhibitory neurons, rods, cones, or retinal ganglion. Rather than using naturally occurring promoters to drive transgene expression, the Roska lab built synthetic promoters from scratch and tested to see if they drove cell type specific expression in the retina.
Four approaches for designing synthetic promoters
To build a library of synthetic promoters, the Roska lab used four different design strategies:
- Strategy #1 (ProA): Create promoters with sequences upstream of the start codon of retinal cell-type specific genes from mice.
- Strategy #2 (ProB): Create promoters using phylogenetically conserved sequences that are upstream of the transcription initiation sites of at least two genes with high expression and high cell-type specificity.
- Strategy #3 (ProC): Create promoters by repeating mouse cell type specific transcription factor binding sites interspaced with random sequences.
- Strategy #4 (ProD): Create promoters with the help of epigenetic analysis and designed using stretches of DNA that were transcriptionally active in retinal cell types in mice.
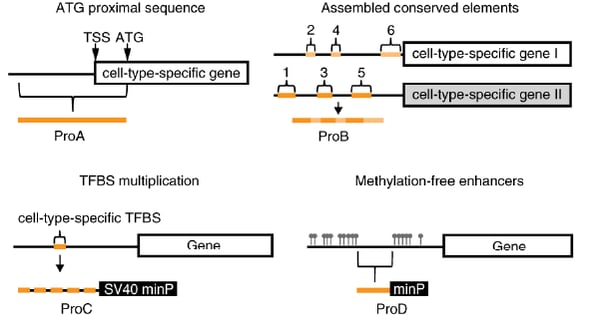 |
| Figure 1: The four strategies used to design synthetic promoters. Legend: TSS: transcription start site. TFBS: transcription factor binding site. Image from Jüttner et al., 2019 with permission. |
In total, this library contained 226 AAVs, each with a different synthetic promoter driving expression of the same transgene: an optogenetic reporter, channelrhodopsin, fused to a fluorescent GFP marker (CatCH-GFP). The lab deposited AAV plasmids containing the active promoters at Addgene.
Performance of synthetic promoter AAVs in mice
All of these synthetic promoter AAVs were first injected into the eyes of mice to test for cell type-specific expression of GFP. Half (113) expressed in retinal cells four weeks post-injection and 14% had targeted expression in specific cell types (ex: rods or cones) or cell classes (ex: photoreceptors which encompassed both rods and cones).
Targeted expression was defined as meeting at least one of the following criteria:
- More than 90% of expression occurred in one type of cell
- More than 50% of expression occurred in one type of cell, with the remaining expression occurring in only Müller glia cells, a common contamination in AAV targeting
- More than 70% of expression was from a single class of cells
Of the four different promoter designs, promoters from strategy #4 had the highest rate of targeted expression. Surprisingly, <1% of synthetic promoters replicated the expression specificity of the wild-type promoters they were derived from. Synthetic promoters did, however, tend to loosely match the specificity of their source genes, i.e. if a synthetic promoter expressed in cells on the outer part of the retina, it was more likely to have borrowed elements from a natural promoter that was active in the outer part of the retina.
Find the AAV synthetic promoter plasmids!
Comparing synthetic promoters performance in mouse, monkey, and human retinas
Next, the 113 AAV promoters that were active in mouse retinas were injected into macaques and postmortem human retinas. About 25% of these AAV synthetic promoters successfully targeted cells in monkey and human retinas. By comparing the performance of promoters across these three species, the Roska lab concluded that a promoter’s ability to target a cell class in mice is not a good predictor for targeting the same cell class in NHPs (R=0.38) and humans (R=0.32), but the odds of that promoter targeting the same cell class in all three species are still greater than random chance. The chances of a promoter expressing in primate and human retinas but not in mice was low (R=0.12), suggesting that mice could be used to eliminate promoters that are also unlikely to express in primates and humans. On the other hand, the ability of an AAV to target a cell class in monkeys was a good predictor for targeting the same cell class in humans (R=0.67).
A database of the microscopy images of all AAVs that reproducibly labeled retinal cells can be found here.
Applications for synthetic promoter AAVs
Besides restricting AAV transgene expression to particular cell types or classes, these synthetic promoters have a few other potential applications:
- Using promoter combinations for targeted expression: While few synthetic promoters targeted specific cell types, pairs of synthetic promoter can be used to restrict expression to a single cell type based on the overlap in cell types both promoters expressed in. The lab tested this application using one promoter to drive expression of Cre-mCherry and a second promoter to drive expression of a Cre-dependent fluorescent CatCH-GFP reporter. Expression of GFP will only result in cells where Cre-mCherry is also expressed. If Cre-mCherry expresses in photoreceptors and horizontal cells, and Cre-dependent-GFP expresses in horizontal cells and ganglion cells, only the horizontal cells transduced by both AAVs would express Cre-mCherry and GFP.
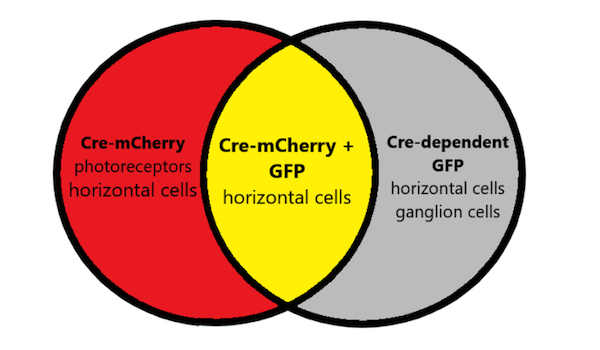
Figure 2: Synthetic promoters can be paired for targeted expression.
This same pairing of synthetic promoters were also used to target multiple cell types and could also be used for sparse labeling of neurons for tracing experiments. - Neuronal tracing experiments: While synthetic promoters with high rates of labeling are ideal of gene therapy applications, promoters that produce sparse labeling would be useful for neuronal tracing experiments where lower levels of labeling makes it easier to trace a neuron’s projections.
- Generating animal models: Transgenic animals allow for the expression of genes in particular tissues, but they are costly and time-consuming to generate, especially for large animals such as primates. Viral vectors with cell-type specific promoters could be an easier way to restrict expression of a gene interest in an animal model.
A toolkit of synthetic AAV promoters
The Roska lab’s synthetic promoter AAVs allows for targeted gene expression in retinal cell types and classes in mice, primate, and human retinas. These synthetic promoters are valuable tools for basic as well as translational research for gene therapy. The Roska lab hopes to design improved versions of their current promoters, as well as more promoters that target cells types missed by their current library (Neff et al., 2019). While tested in retinal cells, the same design principles could be used to create synthetic promoters for cell-type targeted expression in other tissues.
Additional resources on the Addgene blog
- Read more about using AAV for retinal gene therapy
- Find more about precise neuronal targeting using AAV technologies
- Browse all of our viral vector blog posts
Resources at Addgene.org
- Find AAV plasmids
- Find ready-to-use AAV viral preps
- Browse all viral vectors
References
Juettner, J., Szabó, A., Gross-Scherf, B., Morikawa, R.K., Rompani, S.B., Hantz, P., Szikra, T., Esposti, F., Cowan, C.S., Bharioke, A., Patino-Alvarez, C.P., Keleş, Ö., Kusnyerik, A., Azoulay, T., Hartl, D., Krebs, A., Schübeler, D., Hajdú, R.I., Lukáts, Á., Németh, J., Nagy, Z.Z., Wu, K., Wu, R., Xiang, L., Fang, X., Jin, Z., Goldblum, D., Hasler, P.W., Scholl, H.P., Krol, J., & Roska, B. (2019). Targeting neuronal and glial cell types with synthetic promoter AAVs in mice, non-human primates and humans. Nature Neuroscience, 22, 1345-1356. https://doi.org/10.1038/s41593-019-0431-2
Neff, E.P. An AAV library for retinal cell expression in mouse, macaque, and man. Lab Anim 48, 264 (2019). https://doi.org/10.1038/s41684-019-0380-0
Topics: Viral Vectors, Cell Tracing, AAV






Leave a Comment