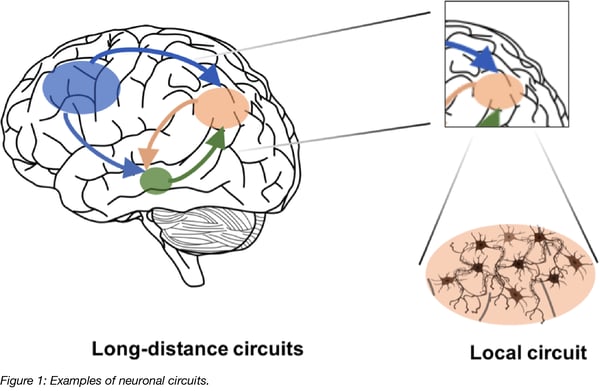
AAV vectors: A platform for delivering neuronal tracers
AAV vectors have been widely used by neuroscientists for some time due to their advantageous characteristics for brain research and their ability to introduce genetic material into neuronal cells. The AAV genome is a single-stranded DNA molecule and AAV vectors are replication deficient (i.e. they cannot easily spread between cells like natural RABV or HSV can). AAV does not have a membrane envelope like lentivirus, but instead is contained by a capsid which is comprised of the viral proteins VP1, VP2, and VP3. To date several hundred variants, or serotypes, have been found naturally or have been genetically engineered to have differences in their capsid proteins. For a brief overview see Addgene’s AAV guide.
AAV particles are relatively small (20 nm) in size which makes them capable of diffusing in the extracellular space; they also have low immunogenicity and cellular toxicity. AAV vectors can be equipped with strong promoters for high and neuron-specific transgene expression. Fluorescent proteins are widely used for tracing and their expression is often made Cre-dependent. If Cre-dependent vectors are used in transgenic animals that express Cre in only specific cell types, for example only dopaminergic neurons, neuronal tracing has the added benefit of only labelling the subset of infected cells that also express Cre. Another advantage of AAV vectors is that there are well-established protocols for their production and purification that yield high titers (see also AAV protocols for production, purification and titration on the Addgene website).
| Tissue | Optimal Serotype |
| CNS | AAV1, AAV2, AAV4, AAV5, AAV8, AAV9 |
| Heart | AAV1, AAV8, AAV9 |
| Liver | AAV7, AAV8, AAV9 |
| Lung | AAV4, AAV5, AAV6, AAV9 |
| RPE (Retinal Pigment Epithelium) | AAV1, AAV2, AAV4, AAV5, AAV8 |
| Skeletal Muscle | AAV1, AAV6, AAV7, AAV8, AAV9 |
Table 1: Common AAV Serotypes and their target tissues (adapted from Addgene's AAV guide)
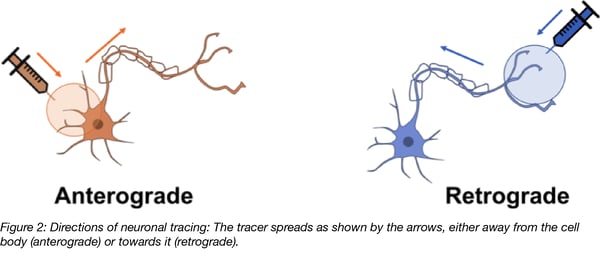
Commonly used serotypes include AAV1 – 9, with most of these serotypes capable of entering neurons (see table 1)(Choi et al., 2005; Taymans et al., 2007; Howard et al., 2008; Watakabe et al., 2015). There are several reports of both retrograde and anterograde intracellular transport of some AAV serotypes, but overall with rather low efficiency. Not all measures of transport efficiency are consistent which may be caused by the diversity of techniques, species, and brain regions used. Evidence for retrograde transport has been found for AAV1, -2, -5, -7, -8 (Taymans et al., 2007), AAV1 (Hollis et al., 2008) AAV6 (Towne et al., 2010), AAV8, -9 (Masamizu et al., 2011); AAV5 (Aschauer et al., 2013). Anterograde transport has been observed for AAV1, -5, -8 (McFarland et al., 2009), and bidirectional transport for AAV1, -8, -9 (Castle et al., 2014). Differences between AAV serotypes may be also linked to differences in viral uptake by cells and to the uncoating step in the viral life cycle.
Using AAV for neuronal tracing
 AAV vectors are the first choice of many neuroscientists, with many groups using them to map neuronal networks. Kohara et al. 2014 traced neurons with an AAV9 vector expressing a Cre-dependent fluorescent marker. Injection into a conditional mutant mouse brain that expressed Cre recombinase only in pyramidal cells of the hippocampal region CA2 allowed for detailed mapping of the neuronal circuitry involved in learning and memory (Kohara et al., 2014; plasmids at Addgene).
AAV vectors are the first choice of many neuroscientists, with many groups using them to map neuronal networks. Kohara et al. 2014 traced neurons with an AAV9 vector expressing a Cre-dependent fluorescent marker. Injection into a conditional mutant mouse brain that expressed Cre recombinase only in pyramidal cells of the hippocampal region CA2 allowed for detailed mapping of the neuronal circuitry involved in learning and memory (Kohara et al., 2014; plasmids at Addgene).
The Gradinaru Lab at the California Institute of Technology generated novel AAVs (Chan et al., 2017) that express one of a few different fluorescent proteins (red, green, blue). If a researcher injects a cocktail of these different AAV vectors into a brain region, the AAV particles will infect neurons at different ratios, making these neurons fluoresce in different colors. An individual neuron can then be traced throughout the brain based on its unique hue (plasmids at Addgene).
A large-scale example of neuronal tracing with AAV vectors is the Allen Mouse Brain Connectivity Atlas, a project to map neuronal connections in the mouse brain. For this project, AAV1 was used as an anterograde tracer and injected at several hundred sites across the entire mouse brain. Traced neurons expressed a fluorescent marker in areas close or distal to the injection site across the entire brain, allowing for an unprecedented number of connections, or the “connectome”, to be visualized in a uniform and standardized way (Oh et al., 2014). Images yielded from this study can be found on the Allen Mouse Brain Connectivity Atlas website.
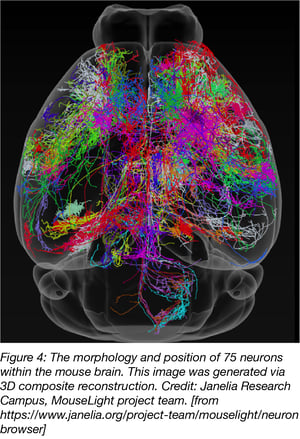
A similar approach was used by researchers from the Janelia Campus for their MouseLight project but neurons were traced with AAV2. Two-Photon imaging was used to generate a 3D finemap of neurons in the mouse brain. MouseLight images can be found on the MouseLight website.
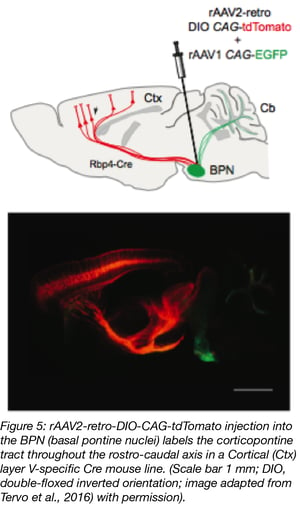 Together with scientists at the University of California, Berkeley, the Janelia Research Campus also developed a new AAV variant, rAAV2-retro, that migrates retrogradely at higher rates than any other AAV serotype (Tervo et al., 2016; plasmid at Addgene). Its efficiency is comparable to conventional dye-based tracing while also having the added advantage of lower toxicity, higher specificity and reduced technical variability compared to chemical tracers. rAAV2-retro also has lower affinity for heparin, which possibly explains why it’s less likely to be sequestered in the extracellular space. In addition, rAAV2-retro’s altered amino acid sequence might facilitate interaction with uptake or intracellular transport machinery. Addgene offers viral preparations of AAV2-retro vectors with a variety of transgene/promoter combinations. Check out our retrograde AAV blog post.
Together with scientists at the University of California, Berkeley, the Janelia Research Campus also developed a new AAV variant, rAAV2-retro, that migrates retrogradely at higher rates than any other AAV serotype (Tervo et al., 2016; plasmid at Addgene). Its efficiency is comparable to conventional dye-based tracing while also having the added advantage of lower toxicity, higher specificity and reduced technical variability compared to chemical tracers. rAAV2-retro also has lower affinity for heparin, which possibly explains why it’s less likely to be sequestered in the extracellular space. In addition, rAAV2-retro’s altered amino acid sequence might facilitate interaction with uptake or intracellular transport machinery. Addgene offers viral preparations of AAV2-retro vectors with a variety of transgene/promoter combinations. Check out our retrograde AAV blog post.
An additional desirable property for network visualization would be the ability to spread trans-synaptically on to subsequent neurons. AAV vectors are replication incompetent and cannot readily be passed on to subsequent cells via replication like RABV or HSV. There are, however, a few reports of trans-synaptic spreading of some AAVs, specifically AAV6 in the retrograde direction and AAV2 in the anterograde direction (Salegio et al., 2013). Zingg et al. 2017 also provide evidence that AAV1 and AAV9 are capable of synaptic spread. The underlying mechanisms of trans-synaptic trafficking are not fully understood and may be a form of transcytosis where the AAV particle is taken up on one side of the cell and released again at another side of the cell after intracellular transport without actually causing infection or leading to transgene expression within the first cell. While there is always a risk that viral particles will diffuse long distances within the extracellular space along an axon, this does not seem to be the case for either AAV1 or AAV9. It should be noted that AAV9 was shown to cross the blood brain barrier by endothelial transcytosis (Foust et al., 2009; Merkel et al., 2017), and these two modes of transport may share common mechanisms. Rare observations like these may be a starting point for future developments in the field of AAV vectors.
Conclusion
An ideal comprehensive toolset for neuronal tracing and network identification requires both antero- and retrograde labelling, synaptic restriction as well as controlled monosynaptic and polysynaptic spreading. AAV vectors cover some of these attributes and their use has allowed for the visualization of single connection networks within the rodent brain. A better understanding of the routes and mechanisms of intracellular transport of AAV could facilitate the development of more powerful AAV serotypes, and perhaps lead to controlled trans-synaptic spread of AAV vectors. Improved neuronal tracing could also be achieved by using optogenetically controlled transgenes, in combination with conditional transgenic mice, to reveal not only the anatomy of neuronal connections, but to visualize neuron connections that are functionally relevant, i.e. activity-dependent neuronal tracing. Apart from mapping neuronal networks, improved AAV vectors could also be powerful tools for the field of gene therapy if they can reach the affected part of the brain in a less invasive manner . For example, in neuromuscular diseases it would be desirable to achieve transgene delivery by targeting peripheral structures (such as injection into muscle) as opposed to direct intracerebral application, which is more invasive and has a much higher risk of complications.
References
Aschauer, D.F., Kreuz, S., and Rumpel, S. (2013). Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One 8:e76310. PubMed PMID: 24086725. PubMed Central PMCID: PMC3785459.
Beier, K.T., Saunders, A., Oldenburg, I.A., Miyamichi, K., Akhtar, N., Luo, L., Whelan, S.P., Sabatini, B., and Cepko, C.L. (2011). Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. Proc Natl Acad Sci USA 108, 15414–15419. PubMed PMID: 21825165. PubMed Central PMCID: PMC3174680.
Buttry, J.L., and Goshgarian, H.G. (2015). WGA-Alexa Transsynaptic Labeling in the Phrenic Motor System of Adult Rats: Intrapleural Injection versus Intradiaphragmatic Injection. J Neurosci Methods 241, 137–145. PubMed PMID: 25555356. PubMed Central PMCID: PMC4324159.
Castle, M.J., Gershenson, Z.T., Giles, A.R., Holzbaur, E.L., and Wolfe, J.H. (2014). Adeno-associated virus serotypes 1, 8 and 9 share conserved mechanisms for anterograde and retrograde axonal transport. Hum Gene Ther 25, 705–720. PubMed PMID: 24694006. PubMed Central PMCID: PMC4137353.
Chan, K.Y., Jang, M.J., Yoo, B.B., Greenbaum, A., Ravi, N., Wu, W.-L., Sánchez-Guardado, L., Lois, C., Mazmanian, S.K., Deverman, B.E., and Gradinaru, V. (2017). Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 20(8), 1172–1179. PubMed PMID: 28671695. PubMed Central PMCID: PMC5529245.
Choi, V.W., McCarty, D.M., and Samulski, R.J. (2005). AAV Hybrid Serotypes: Improved Vectors for Gene Delivery. Curr Gene Ther 5(3), 299–310. PubMed PMID: 15975007. PubMed Central PMCID: PMC1462937.
Dietzschold, B., Li, J., Faber, M., and Schnell, M. (2008). Concepts in the pathogenesis of rabies. Future Virol 3(5), 481–490. PubMed PMID: 19578477. PubMed Central PMCID: PMC2600441.
Foust, K.D., Nurre, E., Montgomery, C.L., Hernandez, A., Chan, C.M. and Kaspar B.K. (2009). Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol 27, 59–65. PubMed PMID: 19098898. PubMed Central PMCID: PMC2895694.
Hollis, E. R. 2nd, Kadoya, K., Hirsch, M., Samulski, R. J., and Tuszynski, M. H. (2008). Efficient retrograde neuronal transduction utilizing self-complementary AAV1. Mol Ther 16, 296–301. PubMed PMID: 18223548.
Howard, D.B., Powers, K., Wang, Y., and Harvey, B.K. (2008). Tropism and toxicity of adeno-associated viral vector serotypes 1,2,5,6,7,8,9 in rat neurons and glia in vitro. Virology 372(1), 24–34. PubMed PMID: 18035387. PubMed Central PMCID: PMC2293646.
Jin, J., and Maren, S. (2015). Prefrontal-Hippocampal Interactions in Memory and Emotion. Front Syst Neurosci 9, 170. PubMed PMID: 26696844. PubMed Central PMCID: PMC4678200.
Kohara, K., Pignatelli, M., Rivest, A.J., Jung, H.-Y., Kitamura, T., Suh, J., Frank, D., Kajikawa, K., Mise, N., Obata, Y., Wickersham, I.R., and Tonegawa S. (2014). Cell type-specific genetic and optogenetic tools reveal novel hippocampal CA2 circuits. Nat Neurosci 17(2), 269–279. PubMed PMID: 24336151. PubMed Central PMCID: PMC4004172.
Kristensson, K., and Olsson, Y. (1971). Retrograde axonal transport of protein. Brain Res 29, 363–365. PubMed PMID: 4107258.
Lo, L., and Anderson, D.J. (2011). A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron 72(6), 938-950. PubMed PMID: 22196330. PubMed Central PMCID: PMC3275419.
Maday, S., Twelvetrees, A.E., Moughamian, A.J., and Holzbaur, E.L.F. (2014). Axonal Transport: Cargo-Specific Mechanisms of Motility and Regulation. Neuron 84(2), 292–309. PubMed PMID: 25374356. PubMed Central PMCID: PMC4269290.
Masamizu, Y., Okada, T., Kawasaki, K., Ishibashi, H., Yuasa, S., Takeda, S., Hasegawa, I., and Nakahara, K. (2011). Local and retrograde gene transfer into primate neuronal pathways via adeno-associated virus serotype 8 and 9. Neuroscience 193, 249–258. PubMed PMID: 21782903.
McFarland, N.R., Lee, J.S., Hyman, B.T., and McLean, P.J. (2009). Comparison of transduction efficiency of recombinant AAV serotypes 1, 2, 5 and 8 in the rat nigrostriatal system. J Neurochem 109, 838–845. PubMed PMID: 19250335. PubMed Central PMCID: PMC2698947.
Merkel, S.F., Andrews, A.M., Lutton, E.M., Mu, D., Hudry, E., Hyman, B.T., Maguire, C.A. and Ramirez S.H. (2017). Trafficking of adenoassociated virus vectors across a model of the blood-brain barrier; a comparative study of transcytosis and transduction using primary human brain endothelial cells. J Neurochem 140, 216–230. PubMed PMID: 27718541. PubMed Central PMCID: PMC5298820.
Oh, S.W., Harris, J.A., Ng, L., Winslow, B., Cain, N., Mihalas, S., Wang, Q., Lau, C., Kuan, L., Henry, A.M., Mortrud, M.T., Ouellette, B., Nguyen, T.N., Sorensen, S.A., Slaughterbeck, C.R., Wakeman, W., Li, Y., Feng, D., Ho, A., Nicholas, E., Hirokawa, K.E., Bohn, P., Joines, K.M., Peng, H., Hawrylycz, M.J., Phillips, J.W., Hohmann, J.G., Wohnoutka, P., Gerfen, C.R., Koch, C., Bernard, A., Dang, C., Jones, A.R., and Zeng, H. (2014). A mesoscale connectome of the mouse brain. Nature 508, 207–214. PubMed PMID: 24695228. PubMed Central PMCID: PMC5102064.
Roberts, T. F., Hisey, E., Tanaka, M., Kearney, M., Chattree, G., Yang, C. F., Shah, N.M., and Mooney, R. (2017). Identification of a motor to auditory pathway important for vocal learning. Nat Neurosci 20(7), 978–986. PubMed PMID: 28504672. PubMed Central PMCID: PMC5572074.
Salegio, E.A., Samaranch, L., Kells, A.P., Mittermeyer, G., San Sebastian, W., Zhou, S., Beyer, J., Forsayeth, J., and Bankiewicz, K.S. (2013). Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene Ther 20(3), 348–352. PubMed PMID: 22418061. PubMed Central PMCID: PMC3381869.
Schmued L.C., and Fallon J.H. (1986). Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res 377, 147–54. PubMed PMID: 2425899.
Schwab M.E., Javoy-Agid F., and Agid Y. (1978). Labeled wheat germ agglutinin (WGA) as a new, highly sensitive retrograde tracer in the rat brain hippocampal system. Brain Res 152, 145-150. PubMed PMID: 79432.
Schwab, M., Suda, K., and Thoenen, H. (1979). Selective retrograde transsynaptic transfer of a protein, tetanus toxin, subsequent to its retrograde axonal transport. J Cell Biol 82(3), 798–810. PubMed PMID: 92475.
Taymans, J. M., Vandenberghe, L. H., Haute, C. V., Thiry, I., Deroose, C. M., Mortelmans, L., Wilson, J.M., Debyser, Z., and Baekelandt, V. (2007). Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7 and 8 in mouse brain. Hum Gene Ther 18, 195–206. PubMed PMID: 17343566.
Tervo, D.G.R., Huang, B.-Y., Viswanathan, S., Gaj, T., Lavzin, M., Ritola, K.D., Lindo, S., Michael, S., Kuleshova, E., Ojala, D., Huang, C.C., Gerfen, C.R., Schiller, J., Dudman, J.T., Hantman, A.W., Looger, L.L., Schaffer, D.V., and Karpova, A.Y. (2016). A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92(2), 372–382. PubMed PMID: 27720486. PubMed Central PMCID: PMC5872824.
Thanos, S., Vidal-Sanz M., and Aguayo A.J. (1987). The use of rhodamine-B-isothiocyanate (RITC) as an anterograde and retrograde tracer in the adult rat visual system. Brain Res 406(1-2), 317-321. PubMed PMID: 2436717.
Towne, C., Schneider, B.L., Kieran, D., Redmond, D.E.Jr., and Aebischer, P. (2010). Efficient transduction of non-human primate motor neurons after intramuscular delivery of recombinant AAV serotype 6. Gene Ther 17, 141–146. PubMed PMID: 19727139.
Ugolini, G., Kuypers, H.G., and Strick, P.L. (1989). Transneuronal transfer of herpes virus from peripheral nerves to cortex and brainstem. Science 243, 89–91. PubMed PMID: 2536188.
Veenman, C.L., Reiner, A., and Honig, M.G. (1992). Biotinylated dextran as an anterograde tracer for single- and double-label studies. J Neurosci Methods 55, 65–78. PubMed PMID: 1381034.
Watakabe, A., Ohtsuka, M., Kinoshita, M., Takaj, M.I., Isa, K., Mizukami, H., Ozawa, K., Isa, T., and Yamamori, T. (2015). Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex, Neurosci Res 93, 144-157. PubMed PMID: 25240284.
Wickersham, I.R., Lyon, D.C., Barnard, R.J.O., Mori, T., Finke, S., Conzelmann, K.-K., Young, J.A., and Callaway, E.M. (2007). Monosynaptic Restriction of Transsynaptic Tracing from Single, Genetically Targeted Neurons. Neuron 53(5), 639–647. PubMed PMID: 17329205. PubMed Central PMCID: PMC2629495.
Zingg, B., Chou, X., Zhang, Z., Mesik, L., Liang, F., Tao, H.W., and Zhang, L.I. (2017). AAV-mediated Anterograde Transsynaptic Tagging: Mapping Input-Defined Functional Neural Pathways for Defense Behavior. Neuron 93(1), 33–47. PubMed PMID: 27989459. PubMed Central PMCID: PMC5538794.
Additional Resources on the Addgene Blog
- Retrograde AAV: Making the journey from axon to nucleus
- Rabies and neuronal tracing
- Important considerations when using AAVs
Resources on Addgene.org
- Find ready-to-use AAV Preps
- Find AAV plasmids
- Browse all viral vectors
Topics: Viral Vectors, Cell Tracing, AAV







Leave a Comment