Horizontal gene transfer (HGT) is the movement of genetic material between organisms. It plays a key role in bacterial evolution and is the primary mechanism by which bacteria have gained antibiotic resistance and virulence. Scientists have studied how HGT occurs in nature and have learned how to introduce genetic materials into cells in the lab.
The introduction of foreign DNA or RNA into bacteria or eukaryotic cells is a common technique in molecular biology and scientific research. There are multiple ways foreign DNA can be introduced into cells including transformation, transduction, conjugation, and transfection. Transformation, transduction, and conjugation occur in nature as forms of HGT, but transfection is unique to the lab. Let’s take a look at these different methods of DNA insertion.
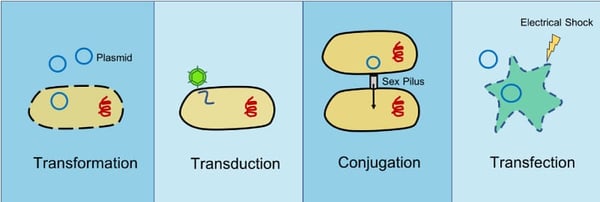
Transformation
Transformation is the uptake of genetic material from the environment by bacterial cells. In nature, this genetic material often comes from adjacent lysed bacteria and can include plasmid DNA or fragmented DNA released into the environment. Various factors promote natural transformation in different bacteria such as growth phase of the cells (Baltrus and Guillemin, 2006) or the presence of specific substances (Meibom et al., 2005).
Though not all bacteria are naturally competent to take up DNA, they can be made competent through chemical manipulation in the lab. This is commonly done using calcium chloride which permeabilizes the cell membrane so the bacteria can easily uptake your plasmid of interest. Scientists can also use electroporation, the application of an electrical charge to cells, to increase cell membrane permeability and thus transformation efficiency. Check out Addgene’s blog to learn about making your own competent cells and our protocols page to learn about bacterial transformation in the lab.
 |
| Gulp! Though bacteria don't exactly "eat" plasmids as depicted in this comic, when a bacterium uptakes a plasmid, the genes on the plasmid can give them different phenotypes. For example, this plasmid encodes a green fluorescent protein gene which makes the bacterium fluoresce green. Comic by Maya Kostman. |
Transduction
Transduction occurs when foreign DNA or RNA is introduced into bacterial or eukaryotic cells via a virus or viral vector.
One example are bacteriophages that attach to bacterial membranes and inject their genetic material into the cell. Once inside, phages can follow one of two different life cycles: lytic or lysogenic. Lytic phages hijack the bacterial hosts machinery to make more viral particles. Eventually the cell lyses releasing the newly formed viral particles that can infect other bacteria. In the lysogenic cycle, the phage’s genetic material is incorporated into the host’s genome at a particular integration site. The integrated phage remains dormant until it is triggered to enter the lytic cycle.
During both of these life cycles bacterial DNA can be accidentally packaged into the newly created phages. Transfer of this DNA to another cell is referred to as transduction. Transferred DNA once inside the infected bacterium can either exist as transient extrachromosomal DNA, like a plasmid, or it can integrate into the host bacterium’s genome through homologous or site directed recombination.
Transduction is a common tool used by scientists to introduce different DNA sequences of interest into a bacterial cell or a host’s genome. To do this scientists commonly use phagemids, a DNA cloning vector that contains both bacteriophage and plasmid properties. The phagemids are packaged into replication-incompetent phage particles with assistance from a ‘helper’ phage prior to transduction.
Scientists also use transduction to introduce foreign DNA into eukaryotic cells, like mammalian cell lines. This can be done with lentiviral and Adeno Associated Viruses (AAV). Lentiviral and AAV can be used to create both transient cell lines, where a gene of interest is expressed but not integrated into the genome and stable cell lines, where foreign DNA is incorporated into the cell’s genome and is thus passed down through cell division. You can find all kinds of different lentiviral and AAV plasmids as well as ready-to-use viral preparations at Addgene. For more information on viral vectors, including transduction download our Viral Vectors 101 eBook.
Bacterial conjugation
Conjugation was the first extensively studied method of gene transfer and was discovered in 1946 by Joshua Lederberg and Edward Tatum when they observed genetic recombination between two nutritional deficient E. coli strains that resulted in a wild type E. coli (Griffiths et al., 2000).
During conjugation, genetic material is transferred from a donor bacterium to a recipient bacterium through direct contact. The donor bacterium contains a DNA sequence called the Fertility factor (F-factor). The F-factor is found on an episome, a piece of DNA that can replicate on its own or be integrated within a bacterial chromosome and allows the donor bacterium to make a small “bridge” or sex pilus that attaches to the recipient cell drawing it close. Once in contact the donor can transfer genetic material to the recipient bacterium. The genetic material transferred is commonly a plasmid and can infer genetic advantages such as antibiotic resistance.
 |
| Bacterial conjugation as explained by the "I made this" meme! Comic by Maya Kostman. |
Transfection
Unlike the last three methods which can be used in prokaryotes, transfection is only done in eukaryotic cells. Transfection is the process by which foreign DNA is deliberately introduced into a eukaryotic cell through non-viral methods including both chemical and physical methods in the lab. Chemicals like calcium phosphate and diethylaminoehtyl (DEAE)-dextra neutralize or even impart an overall positive charge on DNA molecules so that it can more easily cross the negatively charged cell membrane. Physical methods such as electroporation or microinjection actually pokes holes in the cell membrane so DNA can be introduced directly into the cell. Microinjection requires the use of a fine needle to deliver nucleic acids to individual cells. Electroporation on the other hand uses electrical pulses to create transient pores in the cell membrane that genetic material can pass through.
If you are starting any molecular biology experiment check out Addgene’s protocol page, which has both protocols and videos for techniques in basic molecular biology, plasmid cloning, and viruses.

References
Baltrus, David A., and Karen Guillemin. "Multiple phases of competence occur during the Helicobacter pylori growth cycle." FEMS microbiology letters 255.1 (2006): 148-155. PubMed PMID: 16436074.
Griffiths AJF, Miller JH, Suzuki DT, et al. Bacterial Conjugation. In An Introduction to Genetic Analysis. 7th edition (2000).
Meibom, Karin L., et al. "Chitin induces natural competence in Vibrio cholerae." Science 310.5755 (2005): 1824-1827. PubMed PMID: 16357262.
Additional resources on the Addgene blog
- Read all of our Plasmids 101 blog posts
- Check out all of our blog posts on plasmid cloning
Resources on Addgene.org
- Read our protocols for plasmid cloning
- Find our molecular biology reference here
- Find molecular biology protocols here
Topics: Plasmids 101, Plasmids





Leave a Comment