When facing a cloning project, scientists are no longer limited to traditional restriction enzyme cloning. Instead, you can choose a molecular cloning technique that will work well with a given set of resources, time, and experimental needs. Since its invention in the late 1990s, Gateway cloning technology has become very popular as a rapid and highly efficient way to move DNA sequences into multiple vector systems. With the appropriate entry and destination vectors, one can use Gateway to clone a gene of interest into a variety of expression systems. Keep reading to learn more about the Gateway cloning method and its advantages.
An introduction to Gateway technology
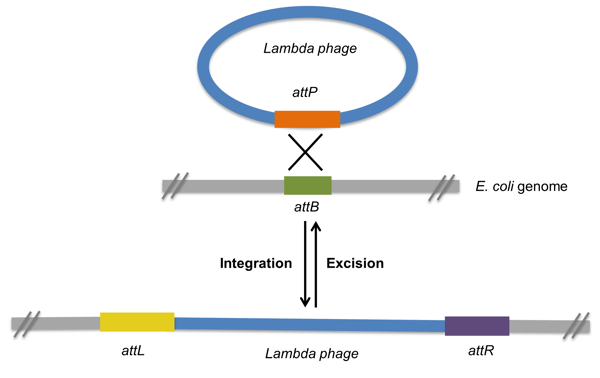
The Gateway cloning method, developed by Invitrogen, is an in vitro version of the integration and excision recombination reactions that take place when lambda phage infects bacteria. In vivo, these recombination reactions are facilitated by the recombination of attachment sites from the phage (attP) and the bacteria (attB). As a result of recombination between the attP and attB sites, the phage integrates into the bacterial genome flanked by two new recombination sites (attL-left- and attR-right-, Figure 1). Under certain conditions, the attL and attR sites can recombine, leading to the excision of the phage from the bacterial chromosome and the regeneration of attP and attB sites.
 |
|
Figure 1: Lambda phage integration and excision reactions. Recombination of attP and attB sites creates attL and attR sites. |
Gateway vectors contain modified versions of the att sites so that scientists can easily clone in their desired DNA sequences. Gateway technology relies on the two reactions described below:
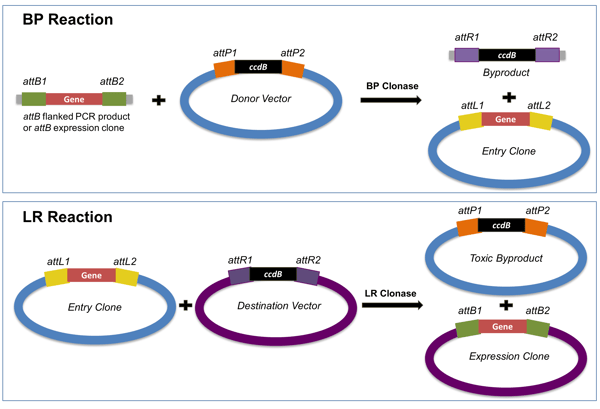
The BP Reaction takes place between the attB sites flanking the insert and the attP sites of the donor vector. This reaction is catalyzed by the BP Clonase enzyme mix and generates the entry clone containing the DNA of interest flanked by attL sites. As a byproduct of the reaction, the ccdB gene is excised from the donor vector.
 |
|
Figure 2: The Gateway system adopts phage integration into the BP and LR reactions. The BP reaction creates an attL-flanked entry clone. The LR reaction creates an expression clone with all of the components necessary for gene expression. |
The LR Reaction takes place between the attL sites of the generated entry clone and the attR sites of the destination vector. This reaction is catalyzed by the LR Clonase enzyme mix. As a result, an expression clone with the DNA of interest flanked by attB sites is generated. As in the BP reaction, a DNA fragment containing the ccdB gene is excised from the destination vector.
Once the BP and/or LR reactions are performed, the next step is to transform competent E. coli cells and select the positive clones. The entry clone and destination vector carry different antibiotic resistance markers (indicated here by plasmid color), allowing you to easily select for the expression clone. You will also need to use a E. coli strain sensitive to CcdB (e.g. DH5α, TOP10, Mach1). The ccdB gene is present in the donor vectors and the destination vectors prior to recombination, and it is exchanged with the gene of interest during the BP or LR reactions. Since the CcdB protein inhibits the growth of CcdB sensitive E. coli strains, most colonies should contain the desired, recombined construct. Read our recent Plasmids 101 Post on CcdB for more information.
How to clone using Gateway technology
To better understand the process, we’ll walk through an example experiment where we might use Gateway cloning to generate our desired constructs: lentiviral expression of the human KRAS gene in mammalian cells.
STEP 1: Generate an Entry Clone
There are a few different ways to generate our desired entry clone - human KRAS flanked by attL sites.
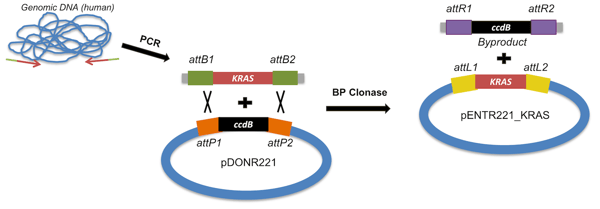
Method A: recombination of an attB-PCR product or plasmid with an attP donor vector. In this case, we would use PCR to add attB sites to either end of the KRAS coding sequence. If you choose this strategy, it’s important to include the proper protein expression elements (ribosome recognition sequences, start codon, stop codons, reading frame considerations, etc). This video demonstrates how to use the Snapgene program to design Gateway plasmids.
 |
|
Figure 3: Method A to create an entry clone: recombination of an attB-flanked PCR product with an attP-containing donor vector. |
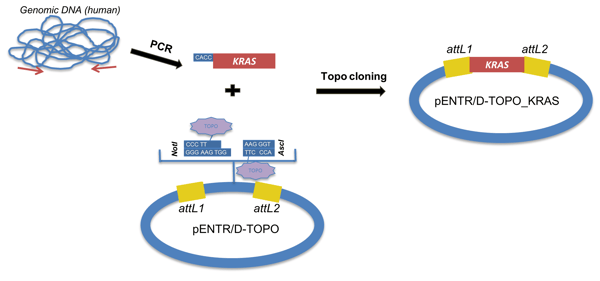
Method B: TOPO-cloning of the desired insert into an attL-entry-TOPO vector. TOPO cloning adds short end(s) to facilitate cloning into an attL-containing entry vector.
 |
|
Figure 4: Method B to create an entry clone: TOPO cloning the insert into an attL-containing entry vector. |
Method C: Restriction cloning of a restriction enzyme fragment containing the DNA of interest and a attL-entry vector. This fragment is inserted in a multiple cloning site (MCS) of an attL-containing entry vector.
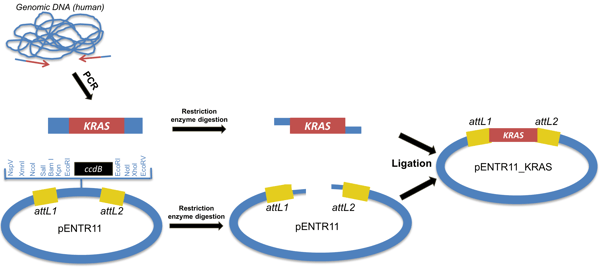 |
|
Figure 5: Method C to create an entry clone: Restriction cloning the inset into an attL-containing entry vector. |
Pro Tip: Addgene also has ready-made entry clones available for many popular genes, including Hs.KRAS4a. Use our website to search for your favorite gene!
STEP 2: Generate an Expression Clone
When making the expression clone, it is important to choose the destination vector that best fits your experiment. This choice will depend on a number of factors, like your organism, desired expression level, and experimental purpose. For mammalian lentiviral expression, we could use a vector like pLenti CMV Puro DEST (w118-1) or the doxycycline-inducible pLIX_403. The chosen attR destination vector will recombine with the attL-entry clone to create the expression clone.
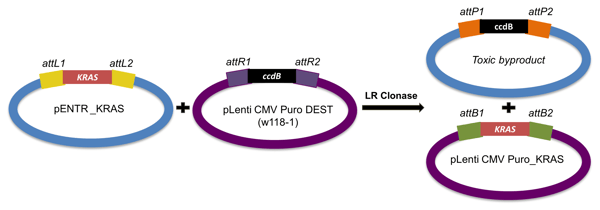 |
|
Figure 6: Generating an expression clone. The reaction between the entry clone and destination vector creates two products: the desires expression clone and a byproduct containing the ccdB gene. Since the ccdB product is toxic to the cell, Gateway cloning efficiency can reach >99%. |
STEP 3: Express your Gene of Interest!
Be sure to verify the integrity of your expression clone via sequencing or restriction digest! Then, you can transform or transfect the cells that you want to use for your experiments and verify that your construct is functional.
Advantages of the Gateway cloning method
Compatibility and flexibility
Once you generate the entry clone with your DNA sequence of interest, you can move this DNA fragment across any expression system in just one recombination step. Addgene’s ready-made entry clones can be used with a large variety of plasmids.
Speed
The Gateway system enables the generation of the expression construct in only 1 day, as opposed to 2+ days with traditional restriction and ligation cloning. It is also possible to set up the BP and LR reactions in the same tube, speeding up the cloning of the attB-PCR products directly into destination vectors. The cloning process is simple - no restriction, ligation or gel purification steps are required!
Multiple fragment cloning
You can use Gateway cloning to insert multiple DNA fragments into many vectors at once in the same tube. You can clone up to 4 DNA fragments, in a specific order and orientation, in one tube, into one Gateway vector to produce the desired expression clone. This is possible thanks to the Gateway vectors’ design. They have modified versions of the attB, P, L and R sites that recombine very specifically and directionally: attB1 sites react only with attP1 sites; attB2 only with attP2, attL1 only with attR1; attL2 only with attR2, and so on. Take a look at some of the Gateway Multisite plasmids available at Addgene, including the Frew Lab Multiple Lentiviral Expression Systems (MuLE) Kit, the MultiSite Gateway cloning kit, and MultiSite Gateway plasmids.
Constant reading frame
When you move a DNA fragment from one Gateway vector to another, the inserted DNA fragment stays in frame.
High efficiency
The positive (antibiotic) and negative (CcdB) selection markers used for Gateway Cloning can increase cloning efficiency to >99%.
Universality
All types of DNA fragments may be cloned: PCR fragments, cDNA or Genomic DNA and is available for all kind of organisms from mammals to E. coli.
Glossary of Gateway cloning vectors
| Vector type | Vector features | Purpose |
| Donor vector | attP sites for recombination; ccdB gene for negative selection | Used to clone attB-flanked genes of interest to generate entry clones |
| Entry vector | attL sites for recombination | Used to generate entry clones by TOPO cloning or by Restriction Cloning |
| Destination vector | attR sites for recombination; ccdB gene for negative selection; elements to express the gene of interest in the appropriate system | Recombines with the entry clone to generate an expression clone |
Ready to try out Gateway cloning?
Many scientists around the world have generated and deposited their own Gateway-compatible plasmids with Addgene. These can be used to express genes in a variety of model organisms. Use the links below to find Gateway plasmids for your organisms of interest:
Find Gateway cloning plasmids here!
References
1. Chee JY, Chin CF (2015) Gateway Cloning Technology: Advantages and Drawbacks. Clon Transgen 4:138. doi:10.4172/2168-9849.1000138
2. Hartley JL. Use of the Gateway System for Protein Expression in Multiple Hosts. Curr Protoc Protein Sci. 2003 Feb;Chapter 5:Unit 5.17. PubMed PMID:18429245.
3. Ptashne, M. (1992). A Genetic Switch: Phage (Lambda) and Higher Organisms (Cambridge, MA: Cell Press).
Additional Resources on the Addgene Blog
- Try Out FastCloning
- Learn About Methylation and Restriction Enzymes
- Use Colony PCR to Quickly Verify Your Clones
Resources on Addgene.org
- Find Plasmid Cloning Protocols
- Watch Our Diagnostic Restriction Digest Video
- Check Out Our Molecular Biology Reference Pages
Topics: Plasmids 101, Plasmid Cloning, Plasmids






Leave a Comment