
While lentiviral vectors are popular gene delivery tools, producing lentivirus, can pose certain challenges. Whether choosing a system that is the best fit for the experiment, trying to produce virus of a usable titer, or fine-tuning selection and expression in your target cell line, researchers often find themselves faced with a roadblock. In this post, we will provide an overview of some of the common challenges associated with producing and using lentivirus and offer some tips and tricks for overcoming these hurdles.
Talking 'bout my (lentiviral) generation
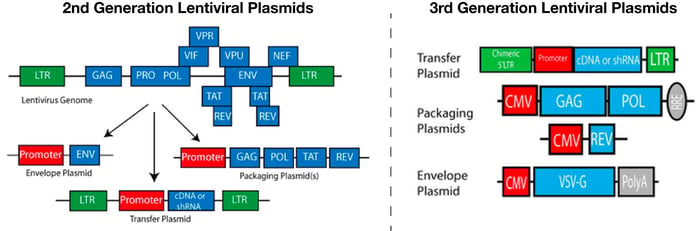
The first step in delivering genes via lentivirus is deciding what packaging system is best for a particular experiment. The newer generation lentiviral packaging systems were designed to prevent the formation of replication competent lentivirus (RCL). Replication competence can arise from recombination events during viral production and poses a biosafety risk to users. To reduce the risk of such events, newer lentiviral packaging systems divide the viral elements among multiple plasmids. Second generation packaging systems use 3 plasmids; an envelope plasmid that usually encodes VSV-G, a packaging plasmid containing structural genes and a transfer plasmid carrying the transfer gene. By dividing the viral elements across multiple plasmids, the risk of recombination and consequently, the generation of RCLs is greatly reduced. Third generation packaging systems further split the viral genome by removing the viral Rev gene from the packaging plasmid and incorporating it into a fourth plasmid. In addition, the promoter of the 5’ long terminal repeat (LTR) is mutated in third generation systems such that it no longer requires the viral transactivator, Tat. For more information on the different generations of lentiviral packaging systems please see Addgene’s Lentiviral Guide.
While the third generation packaging systems offer an extra level of safety, they also require a fourth plasmid. The requirement for additional DNA can impact the transfection efficiency of the packaging cell line and thereby affect the titer of the virus. Additional plasmids will also affect experiment optimization. Before beginning an experiment, it is recommended that you try a few different ratios of transfer, envelope, and packaging plasmids in order to obtain the best titers. Requiring an additional plasmid for transfection can make these optimization experiments a bit more challenging. With these considerations in mind, when planning an experiment users should weigh the benefits of enhanced biosafety versus a potential hit to titer; the level of BL2 experience of end-users, size of the transgene, and downstream applications should all be considered when choosing the correct system.
Recently, a fourth generation packaging system has become available through Clontech. This system improves safety by dividing the viral genome into additional plasmids thereby increasing the number of recombination events that are required to generate RCL. To overcome the titering hurdles of third generation packaging systems, this system incorporates the Tet-off transcriptional activation system to drive high levels of expression of certain viral components; according the manufacturer these changes result in yields up to 25x higher than other available systems.
Producing lentivirus: it's all about the T's (transfection and titers)
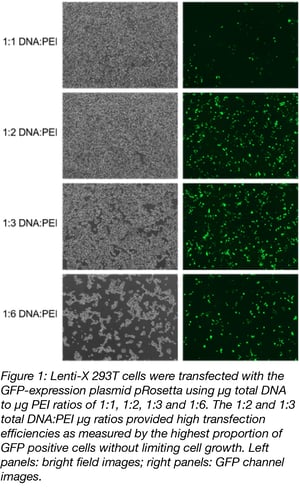 To produce lentivirus, the plasmids encoding the structural elements, envelope, and transfer gene are transfected into a packaging cell line, usually a derivative of 293T. Packaging cells are incubated and after several days the supernatant is collected, filtered, and titered. A major obstacle in obtaining usable lentivirus is producing a high enough concentration, or titer, for your experiment. To improve titer, first optimize transfection conditions. As mentioned above, plasmid ratios can be adjusted to enhance titer. In addition the transfection method (i.e. polyethyleneimine, calcium phosphate or liposomal methods), the volume of the reaction, and the timing and number of harvests will all impact the titer. Importantly, optimized conditions for one transfer plasmid are not always the best for others. For example, in our hands we find that a double harvest approach at 48 and 72h produces high titer virus for transfer plasmids with small inserts (such as CRISPR guides) while a single 72h harvest is best for larger genes such as Cas9. See figure 1 for an example of how changing transfection ratios can affect transfection efficiency.
To produce lentivirus, the plasmids encoding the structural elements, envelope, and transfer gene are transfected into a packaging cell line, usually a derivative of 293T. Packaging cells are incubated and after several days the supernatant is collected, filtered, and titered. A major obstacle in obtaining usable lentivirus is producing a high enough concentration, or titer, for your experiment. To improve titer, first optimize transfection conditions. As mentioned above, plasmid ratios can be adjusted to enhance titer. In addition the transfection method (i.e. polyethyleneimine, calcium phosphate or liposomal methods), the volume of the reaction, and the timing and number of harvests will all impact the titer. Importantly, optimized conditions for one transfer plasmid are not always the best for others. For example, in our hands we find that a double harvest approach at 48 and 72h produces high titer virus for transfer plasmids with small inserts (such as CRISPR guides) while a single 72h harvest is best for larger genes such as Cas9. See figure 1 for an example of how changing transfection ratios can affect transfection efficiency.
For some transgenes, transfection optimization simply is not enough to get a sufficient titer; this is often the case with large genes. This is due to inefficient packaging of large inserts; a study by Kumar et al. found that in some cases, titers can drop 10-fold for every 2kb increase in insert size. To overcome this, lentivirus can be concentrated by a variety of methods such as ultracentrifugation, centrifugation on a sucrose gradient, spin columns, and polyethylene glycol (PEG) precipitation. All of these methods ultimately isolate the viral particles allowing you to concentrate in a smaller volume. Consequently, you will end up with a considerably lower volume than with the unconcentrated prep. For example, at Addgene we typically concentrate 100-fold meaning that a 10 cm2 dish preparation of virus will only yield 150 μl of material as opposed to the 15 ml collected from an unconcentrated prep.
With this in mind, if the experiment requires a certain volume of virus, consider scaling up by either increasing the number of 10 cm2 dishes or increasing the vessel size. Opting for a larger vessel, such as a 3 or 5-layer flask will require some initial optimization; cell number, media volume, and plasmid concentrations will need to be optimized prior to starting. We recommend calculating the ratio of the surface area of the larger vessel to the 10 cm2 dish and adjusting the cell number, plasmid concentration, and experimental volumes accordingly. While you may need to make some final adjustments, this is generally a good place to start. If you don’t anticipate that you will need large volumes of lentivirus routinely, you may opt for simply increasing the number of 10 cm2 dishes. While this option is attractive because the same procedure for a single 10 cm2 dish can be easily applied to multiple dishes, remember that handling multiple dishes will take longer and may increase the risk of mistakes or contamination.
Finally, choice of concentration method depends of a variety of factors such as resources available, cost and downstream applications. For example, while the PEG precipitation method is relatively inexpensive and does not require an ultracentrifuge, PEG will precipitate all proteins in a solution, not just the lentivirus; consequently, this may not be the best method when pure virus is required downstream.
When assessing lentiviral titer, don't compare apples to oranges
After choosing a packaging system and producing lentivirus, it is time to titer. One important consideration when titering a lentivirus is the cell line that is used for titering. Typically researchers will choose a relatively permissive cell line for titering in order to obtain the best titer possible. While this information provides a great starting point, it may not translate to the target cell line. To overcome this issue, we recommend using a range of doses of lentivirus when transducing a target cell line. One should also consider the specifics of the titering transduction experiment. Media choice, reaction vessel, dose of virus, volume and timing of transduction will all impact the recorded titer and should be taken into consideration when transducing the target cell line. For more tips on lentiviral titering please see our blog post.
Between choosing the best lentiviral system, producing virus of an acceptable titer, and successfully transducing a cell line of interest a lot can go wrong. We hope that we have provided you with a few ideas to consider if you do run into a roadblock and encourage you to check out the lentiviral protocols and blog posts on Addgene’s website for additional resources.
References
Kumar M, Keller B, Makalou N, Sutton RE. Systematic determination of the packaging limit of lentiviral vectors. Hum Gene Ther. 2001 Oct 10;12(15):1893-905. PubMed PMID: 11589831.
Jiang W. et. al.. An optimized method for high-titer lentivirus preparations without ultracentrifugation. Sci Rep. 2015; 5:13875. PubMed PMID: 26348152. PubMed Central PMCID: PMC4562269.
Additional Resources on the Addgene Blog
- Your lentiviral FAQs answered
- Tips for titering your lentivirus
- Visit the viral vector featured topic page
Resources on Addgene.org
- Lentiviral Guide
- Find lentivirus plasmids
- Browse all viral vectors
Topics: Viral Vectors, Viral Vectors 101, Viral Vector Protocols and Tips, Retroviral and Lentiviral Vectors






Leave a Comment