This post was updated on March 21, 2018.
Most of the time, plasmid prepping is a breeze. You get your stab from Addgene, streak for single colonies, sub-culture, and prep with a DNA prep kit or your lab's favorite in-house protocol. DNA yields for this procedure are typically in excess of 100 ng/ul, more than enough DNA to verify your plasmid via sequencing or restriction digest.
Chances are, you’ll even have DNA left over for other applications, like PCR, cloning, transfection, or long-term storage. But what about those pesky situations where your plasmid yield is sub-optimal? If you are consistently getting sub-optimal plasmid yields from your prep, try our tips below!
How to optimize your plasmid prep
We'll discuss the following techniques to increase plasmid yield. Keep in mind that you might need to use multiple techniques for a particularly stubborn prep.- Check the bacterial strain
- Increase the volume
- Improve the culture media
- Let it incubate
Check the bacterial strain
Differences between E. coli host strains can impact plasmid yield. Check the bacterial strain to see if additional steps are needed to optimize yield. You might want to include an extra wash step or use a different strain.
For most applications, Addgene uses and recommends DH5𝝰!
This strain consistently gives good, high quality preps due to its endA1 recA1 relA1 genotype, as these mutations improve plasmid stability and yield.
If a plasmid is prone to recombination
Use a strain optimized for plasmids with repeat elements, such as viral plasmids with inverted terminal repeats. We use NEB Stable to transform and prep high quality DNA that contains these elements.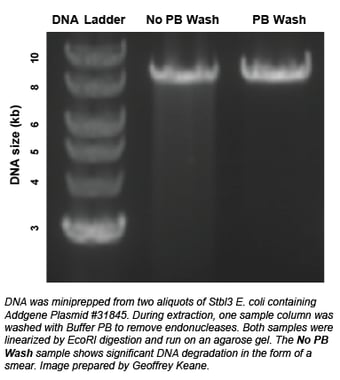
Be aware of endA+ strains
Strains like Stbl3 have been engineered to reduce plasmid recombination, but are also endA+. endA encodes a thermostable periplasmic endonuclease that can sometimes co-purify with your plasmid DNA and lead to degradation of your prep. Many plasmid prep kits recommend performing an extra wash step to remove endonucleases (see figure at right for an example).
Look out for carbohydrates
Certain strains, including HB101 and derivatives, release large amounts of carbohydrates that can inhibit the cell lysis process. To ensure that lysis is complete, use a reagent like Qiagen’s LyseBlue, which uses color to show you if lysis is incomplete.
Protein expression strains are not good for cloning
Bacterial strains adapted for protein purification use the cell’s resources to produce high levels of protein, not plasmid DNA. If you need large amounts of DNA from a plasmid in a protein expression strain, we suggest retransforming the plasmid into a strain meant for DNA purification. It sounds like extra work, but it will likely save time in the long run!
While the choice of strain will ultimately depend on the particular features of your plasmid, it is advisable to check out the genotype of your E. coli to ensure it is suitable for your application. We have previously reviewed many common lab E. coli strains on the blog, and also recommend this handy guide from OpenWetWare for a more extensive listing of common E. coli strains.
Increase the volume
Starting with a larger culture volume can improve plasmid yield. Our lab often doubles the volume of culture used for inoculation if we get inadequate plasmid DNA yields.
Why would volume make a difference? In the simplest of terms, more volume = more cells = more plasmid.
Plasmids can vary in copy number, the number of plasmid copies supported within each bacterial cell in your culture. Copy number is partially specified by a plasmid’s origin of replication, but can also be affected by the plasmid size, nature of the insert, propagation strain, growth conditions, and the material used for inoculation. To further complicate matters, plasmid copy numbers are often ambiguous and should be viewed more as a guideline, since they are typically based on the copy number of the empty plasmid backbone or a researcher’s personal observations. If you are experiencing low yields from a plasmid whose copy number is unknown, it is entirely possible that you are working with a low copy plasmid.
When increasing the culture volume, be aware that a higher volume of lysate passed over the binding matrix could clog the matrix when performing filter based plasmid prepping. Be sure to stay within the recommended range of the filter or you might inadvertently lower the amount of DNA you purify! Additionally, if you've reached your lysate volume limits, the copy number of some origins of replication (e.g. p15A, ColE1) can be increased by adding chloramphenicol to the culture medium.
Improve the culture media
Changing to a richer media or adding supplements can improve your cell density, leading to an improved plasmid DNA yield. Culture conditions play a critical role in plasmid DNA yield. Different plasmids and strains will vary in their optimal growth conditions. For many high copy plasmids, standard LB broth and antibiotic concentrations will work just fine; however, for low copy plasmids or slower growing strains, a change in media may be advantageous.
Reduce the antibiotic concentration
Low copy plasmids produce fewer transcripts of their antibiotic resistance genes and can therefore be cultured in media containing half the normally recommended antibiotic concentration.
Add supplements to the media
You can achieve higher cell density using a nutrient-rich broth such as Terrific Broth, 2xYT, or a home-brew optimized for plasmid yields. Spiking your media with supplements such as magnesium salts, buffering agents, and/or supplying additional carbon sources such as glycerol or glucose (in moderation) may also serve to increase cell density and plasmid yield.
Start with a single, fresh colony
Sub-culturing directly from a frozen glycerol stock or agar stab may lead to loss of the plasmid, and using older plates could increase plasmid loss or mutation.
Let it incubate
Increasing the incubation time can improve the cell density and therefore the plasmid yield. You can optimize your growth time, temperature, and shaking speed to maximize cell density and plasmid yield as these factors are generally, but not perfectly, correlated.
Typical growth times for high copy plasmids in standard growth strains range from 12-16 hours, but cultures with lower copy plasmids may need to be grown for 20 hours or more to achieve maximum plasmid yield. The optimal growth time should be determined for each plasmid/strain combination individually, based on determining the cell density or by harvesting and miniprepping at various time points. Measuring the optical density of the culture can indicate the number of cells present in the given volume.
Let oxygen in!
Make sure your culture is getting the right amount of oxygen for optimal growth, as insufficient gas exchange will prevent your cultures from reaching the desired density. The volume of your flask or culture tube should be at least four times greater than your total culture volume and you should ensure the shaker speed is fast enough for sufficient gas exchange in the overnight culture. Generally, large cultures in flasks may be shaken around 220 RPM, but smaller cultures, especially those in deep-well plates, require a faster shaking speed for proper aeration (260-300 RPM).
37℃ doesn’t work for every plasmid
Certain plasmids and strains grow best at temperatures other than the standard 37℃. This plasmid feature should be indicated in the plasmid information or growth strain instructions from the manufacturer. On Addgene plasmid pages, you may see depositor recommendations to grow a plasmid at 30℃ or room temperature. These recommendations also often require a longer growth time.
Note: Julian Taylor-Parker contributed to the writing of this post.
Additional Resources on the Addgene Blog
- Visit our Plasmids 101 Topic Page
- Learn How to Verify Your Plasmid
- Learn about Common Lab E. coli Strains
Additional Resources on Addgene.org
- Find Info on How to Quantify Your DNA
- Learn to Do a Bacterial Transformation
- Learn How to Perform a Diagnostic Restriction Digest
Additional Resources
- Sigma-Aldrich Introduction to Microbial Media
- BitesizeBio: How to Get Better Plasmid Midiprep Yields
- BitesizeBio: Better Plasmid Midipreps Part II: What Causes Low Yields?
Topics: Plasmids 101, Molecular Biology Protocols and Tips, Plasmids






Leave a Comment