Neuromodulators like dopamine and norepinephrine have important functions in the brain but have been difficult to study without biosensors to directly visualize their activity. In 2019, the first generation of norepinephrine sensors was developed, named GRABNE, which helped researchers visualize norepinephrine (Feng et al., 2019). But this first generation of tools had limited sensitivity and speed and were only available as green probes. The Patriarchi Lab aimed to overcome these limitations by developing and depositing two new norepinephrine indicators: nLightG and nLightR (Kagiampaki et al., 2023).
|
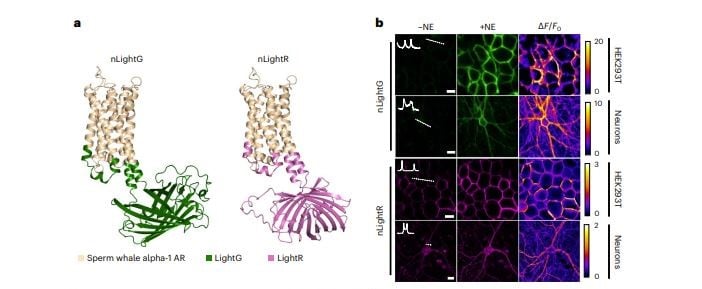
| Figure 1: Properties of nLightG and nLightR. A, Structural models of nLightG (left) and nLightR (right) generated using ColabFold (Mirdita et al., 2022). B, Representative images of HEK293T cells and neurons expressing nLightG or nLightR before/after application of NE (10 μM) and corresponding pixel-wise ΔF/F0 heatmaps. White insets indicate surface expression of the indicators over white dashed lines. Scale bars, 10 μm (HEK293T), 20 μm (neurons). Figure and caption adapted from Kagiampaki et al., 2023. |
nLight
nLight sensors are based on alpha-1a adrenergic receptor; nLightG contains circularly permuted green fluorescent protein (GFP), and nLightR uses a red fluorescent protein, mApple. Kagiampaki et al. found nLight is suitable for in vitro, ex vivo, and in vivo experiments, and nLightG was successfully trialed in two-photon in vivo experiments.
nLight indicators are more sensitive to and selective for norepinephrine and faster to respond than GRABNE. While GRABNE is blocked by alpha2-AR antagonist, nLight sensors are not. Instead, they are blocked by alpha1-AR antagonist. This is important because alpha2-AR can provide inhibitory feedback in response to norepinephrine release, and clinically-used drugs targeting these receptors cannot be studied using GRABNE. They can, however, be studied with the nLight indicators.
Speed
One of the major advantages of this next generation of norepinephrine sensors is their speed. nLightG is activated eight times faster than GRABNE and deactivated three times faster. This enhanced response time allows for more precise measurement of norepinephrine uptake throughout the brain.
Sensitivity
In ex vivo studies on mouse brain slices, nLightG and GRABNE were more sensitive than nLightR, both generating a measurable signal after electrical stimulation with a single pulse, while nLightR gave a measurable signal not at one pulse, but at the second test point, ten pulses. This is in line with the general findings that red fluorescent proteins tend to be less sensitive than green fluorescent proteins. nLightR did perform similarly to GRABNE, and having both colors allows for increased options for multiplexing in experiments, including ones that study alpha2-AR.
Selectivity
Norepinephrine and dopamine are similar molecules, and cross-reactivity is a very common problem when studying norepinephrine. nLight is 28-fold more selective than GRABNE, with both nLightG and nLightR showing significantly increased selectivity for norepinephrine over dopamine. All three sensors, however, did display some reaction to high concentrations of dopamine. This cross reactivity will need to be accounted for in your experimental design.
Next steps
While nLightG and nLightR greatly expand the toolbox for researchers looking to study norepinephrine, the Patriarchi lab continues to work on further improvements. In particular, they’re focused on generating variants with improved affinity and dynamic range of both nLightG and nLightR, and may soon have a red tool which matches the sensitivity of nLightG.
Many thanks to Mike Waring and Tommaso Patriarchi for their assistance with this blog post.
References and resources
References
Feng, J., Zhang, C., Lischinsky, J. E., Jing, M., Zhou, J., Wang, H., Zhang, Y., Dong, A., Wu, Z., Wu, H., Chen, W., Zhang, P., Zou, J., Hires, S. A., Zhu, J. J., Cui, G., Lin, D., Du, J., & Li, Y. (2019). A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine. Neuron, 102(4), 745-761.e8. https://doi.org/10.1016/j.neuron.2019.02.037
Kagiampaki, Z., Rohner, V., Kiss, C., Curreli, S., Dieter, A., Wilhelm, M., Harada, M., Duss, S. N., Dernic, J., Bhat, M. A., Zhou, X., Ravotto, L., Ziebarth, T., Wasielewski, L. M., Sönmez, L., Benke, D., Weber, B., Bohacek, J., Reiner, A., … Patriarchi, T. (2023). Sensitive multicolor indicators for monitoring norepinephrine in vivo. Nature Methods, 20(9), Article 9. https://doi.org/10.1038/s41592-023-01959-z
Mirdita, M., Schütze, K., Moriwaki, Y., Heo, L., Ovchinnikov, S., & Steinegger, M. (2022). ColabFold: Making protein folding accessible to all. Nature Methods, 19(6), Article 6. https://doi.org/10.1038/s41592-022-01488-1
More resources on the Addgene blog
- Fluorescent proteins 101: Fluorescent Biosensors
- Which Fluorescent Microscopy Technique is Best for Me?
- In Living Color: The Skinny on In Vivo Imaging Tools
Resources on Addgene.org
- Find Biosensor Plasmids
- Find Optogenetics Plasmids
- Visit the Fluorescent Protein Guide Pages
Topics: Fluorescent Proteins, Fluorescent Imaging





Leave a Comment