One of the biggest barriers to gene therapy treatment of central nervous system (CNS) diseases is the blood brain barrier (BBB). It’s the BBB’s job to block the entry of pathogens to the CNS, but this also stops viral vectors, such as adeno-associated virus (AAV), from reaching their targets.
The AAV9 serotype can breach the BBB following injection into the bloodstream, but requires a high vector dose and is unable to reach all regions of the CNS. PHP.B, a neurotropic variant of AAV9, is better than AAV9 at gaining access to the CNS of C57BL/6J mice following IV injection but fails to reach the CNS of BALB/cJ mice. These results were surprising because in vivo transduction of AAV serotypes generally tracks between strains of mice. This suggests that a small genetic difference between these two mice might drive their divergent phenotypes. Two labs, the Deverman lab at the Broad Institute and the Wilson lab at the University of Pennsylvania independently searched for this unique sequence of DNA and both identified lymphocyte antigen 6 complex (LY6A) as a receptor for PHP.B.
Read on to learn how the design of PHP.B may have contributed to this mouse-specific performance, why LY6A is an unlikely receptor for PHP.B, and how the Deverman and Wilson labs determined LY6A was a receptor used by PHP.B viruses.
Creating PHP.B, a neurotropic AAV9 variant
PHP.B’s specificity for C57BL/6J mice is likely due to how the Deverman and the Gradinaru labs engineered PHP.B. PHP.B was created by first generating a diverse library of AAV9 mutants that had a small randomized peptide loop inserted in the AAV9 capsid. This mutant library was then screened in vivo in C57BL/6J mice strains to identify variants that reached the CNS following IV injection. Compared to AAV9, PHP.B has a >40-fold increase in CNS transduction and transduces the majority of astrocytes and neurons across multiple CNS regions. PHP.eB, an enhanced version of PHP.B, shows similar transduction efficiency, but at a log lower dose than PHP.B.
A Ly6a variant is linked to PHP.B CNS permissiveness
Both the Deverman and Wilson labs determined LY6A, also known as stem cell antigen-1 (SCA-1), is the receptor used by PHP.B to reach the CNS; yet LY6A is an unlikely candidate. It was discovered as an antigen upregulated on activated lymphocytes and is commonly used to enrich adult mouse hematopoietic stem cells. Its ligand(s) has not been identified. While LY6A or other Ly6 gene family members are linked to viral susceptibility (mouse adenovirus in mouse; HIV1, West Nile, and Influenza in humans), LY6A lacks a defined role in viral susceptibility.
The Deverman and Wilson Labs independently identified LY6A as a receptor for PHP.B by looking for a unique sequence of DNA that was present in mice whose CNS was transduced by PHP.B but absent in mice who were not transduced by PHP.B.
The Deverman Lab took a broad approach and searched for genetic difference between the 36 mouse strains that are part of the Mouse Genome Project. They focused their search on SNPs and small insertions and deletions (indels) most likely to have large phenotypic effects such as those that affected gene expression, mRNA splicing, or protein coding regions. In total, they tested 13 strains, with seven strains identified as permissive and six as non-permissive to PHP.B CNS transduction. Two genes were associated with the PHP.B permissive phenotype, but only one, Ly6a, showed differential protein and RNA expression between the brains of C57BL/6J mice and BALB/cJ mice, suggesting it was a likely player in PHP.B permissiveness.
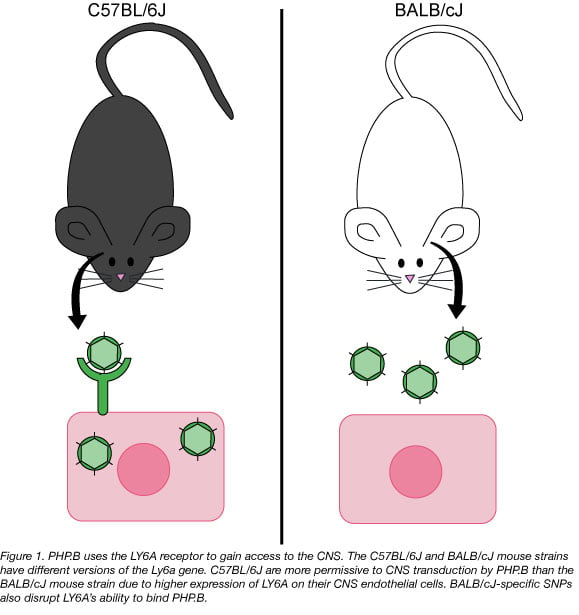 The Wilson Lab also looked for the gene variant responsible for the PHP.B permissive phenotype in mice. They took a classic genetics approach and interbred C57BL/6J and BALB/cJ mice to map the inheritance pattern of PHP.B permissiveness. The first generation of outbred mice (F1) had half the level of CNS PHP.B transduction as their C57BL/6J parent. The second generation (F2) of outbred mice also showed a Mendelian inheritance pattern that suggested there was a single gene that had two codominant versions or alleles that was driving the PHP.B phenotype. Exome sequencing of related hybrid mice narrowed the search to a ~4.5 Mbp region of the mouse chromosome. Of the genes located in this stretch of DNA, Ly6a seemed the most likely candidate due to its strong linkage to the PHP.B phenotype and its expression pattern in the mouse brain.
The Wilson Lab also looked for the gene variant responsible for the PHP.B permissive phenotype in mice. They took a classic genetics approach and interbred C57BL/6J and BALB/cJ mice to map the inheritance pattern of PHP.B permissiveness. The first generation of outbred mice (F1) had half the level of CNS PHP.B transduction as their C57BL/6J parent. The second generation (F2) of outbred mice also showed a Mendelian inheritance pattern that suggested there was a single gene that had two codominant versions or alleles that was driving the PHP.B phenotype. Exome sequencing of related hybrid mice narrowed the search to a ~4.5 Mbp region of the mouse chromosome. Of the genes located in this stretch of DNA, Ly6a seemed the most likely candidate due to its strong linkage to the PHP.B phenotype and its expression pattern in the mouse brain.
Both labs then took similar routes to verify LY6A’s role as a receptor for PHP.B. The Deverman lab started by seeing how changes in LY6A’s expression affected PHP.B’s transduction rates. Knock out of Ly6a in primary C57BL/6J brain endothelial cells decreased transduction rates while overexpression of the C57BL/6J version of Ly6a in 293T cells enhanced PHP.B transduction rates. The group also determined the effect of mice strain specific Ly6a SNPs on PHP.B binding in vitro. They found that when LY6A contained C57BL/6J SNPs, PHP.B could bind it, but when LY6A contained BALB/cJ SNPs, binding was reduced.
The Deverman group then looked to see if PHP.B required the same receptors that its parental AAV9 relied on for transducing cells: galactose and the AAV receptor (AAVR). Cell lines lacking galactose were still transduced by PHP.B but only if Ly6a was expressed. Additionally when AAVR knockout mice were IV injected with PHP.eB, an enhanced version of PHP.B, virus still reached the brain endothelial cells of these mice, although at lower levels than in AAVR wild-type mouse. These results suggested that PHP.B, unlike its parental AAV9, does not require galactose and AAVR to transduce cells.
The Wilson Lab generated results similar to the Deverman Lab when they looked at LY6A protein and RNA expression patterns, the PHP.B permissive phenotype of mouse strains beside C57BL/6J and BALB/cJ, and the effects of overexpressed Ly6a on PHP.B’s transduction of 293T cells. In addition, the Wilson Lab also tested the ability of PHP.B to transduce the CNS of a Ly6a knockout C57BL/6J mouse following IV injection. Not surprisingly, brain transduction was not detected. They also used an ELISA to measure binding of PHP.B to a soluble version of LY6A. They saw binding between PHP.B and the C57BL/6J version of LY6A, but a mutation in PHP.B’s unique capsid loop disrupted binding. Collectively these results suggest that PHP.B uses the LY6A receptor to gain access to the CNS.
The bottom line of viral vector permissiveness
While PHP.B has impressive CNS transduction in some strains of mice, these results are not reproduced when the vector is used in different mouse strains. The in vivo selection for PHP.B in C57BL/6J mouse strains likely contributed to the unintentionally selection of a virus with mouse-strain dependent performance. This surreptitious finding illustrates how the method used to select novel AAV capsid variants could unintentionally limit the utility of candidate capsids.
Are you interested in using AAV-PHP.B vectors? See Supplementary Table 3 in Huang et al. for a list of PHP.B permissive and non-permissive mouse strains. PHP.B plasmids and PHP.eB viral preps are available from Addgene.
References
Holmes, Christina, and William L. Stanford. "Concise review: stem cell antigen‐1: expression, function, and enigma." Stem cells 25.6 (2007): 1339-1347. PubMed PMID: 17379763.
Hordeaux, Juliette, et al. "The GPI-Linked Protein LY6A Drives AAV-PHP. B Transport across the Blood-Brain Barrier." Molecular Therapy 27.5 (2019): 912-921. PubMed PMID: 30819613.
Hordeaux, Juliette, et al. "The neurotropic properties of AAV-PHP. B are limited to C57BL/6J mice." Molecular Therapy 26.3 (2018): 664-668. PubMed PMID: 29428298 PubMed Central PMCID: PMC5911151.
Huang, Qin, et al. "Delivering genes across the blood-brain barrier: LY6A, a novel cellular receptor for AAV-PHP. B capsids." bioRxiv (2019): 538421.
If you’re interested in how PHP.B performed in non-human primates, check out these two references:
- Dayton, Robert D., Mychal S. Grames, and Ronald L. Klein. "More expansive gene transfer to the rat CNS: AAV PHP. EB vector dose–response and comparison to AAV PHP. B." Gene therapy 25.5 (2018): 392. PubMed PMID: 30013186.
- Matsuzaki, Yasunori, et al. "Intravenous administration of the adeno-associated virus-PHP. B capsid fails to upregulate transduction efficiency in the marmoset brain." Neuroscience letters 665 (2018): 182-188. PubMed PMID: 29175632.
If you want to learn more about how PHP.B and PHP.eB were engineered, check out these two references:
- Chan, Ken Y., et al. "Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems." Nature neuroscience 20.8 (2017): 1172. PubMed PMID: 28671695. PubMed Central PMCID: PMC5529245.
- Deverman, Benjamin E., et al. "Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain." Nature biotechnology 34.2 (2016): 204. PubMed PMID: 26829320. PubMed Central PMCID: PMC5088052.
Additional resources on the Addgene blog
- Learn more about PHP.eB, and PHP.S, a peripheral nervous system (PNS) targeting AAV
- Get 1st time AAV user tips from an AAV rookie
- Learn about some considerations when using AAVs
- Learn more about using AAV as a tool for gene expression in mammals.
Resources on Addgene.org
- Search for AAV vectors to use with your research.
- Visit Our AAV Guide Page for info on AAV components, common uses of AAV, and much more!
- Find ready-to-use AAV from Addgene or check out protocols for packaging your own virus
Topics: Viral Vectors, AAV







Leave a Comment