When it comes to using antibodies in the lab, we focus on a lot on the variable domain and not so much on the constant, or Fc, domain. Sure, we all know that the Fc domain provides structure, determines isotypes, and provides a place for secondary antibodies to bind. We also know that changing the Fc domain can impact an antibody's binding affinity (Janda et al., 2016; Torres & Casadevall, 2008) but…does it really do anything?
Fc functions
 The answer is, yes, it does! While the Fc domain’s functions, besides structure and isotype determination, don’t generally affect antibody applications like ELISAs or Western blots, they are key in helping antibodies drive an effective immune response against a pathogen. The Fc domain creates a link between the antibody and other parts of the immune system, including the innate immune response, by binding to Fc receptors on other immune cells. (psst! Need a quick review of the different immune responses? We got you! You can also review antibodies in our Antibodies 101 animation.)
The answer is, yes, it does! While the Fc domain’s functions, besides structure and isotype determination, don’t generally affect antibody applications like ELISAs or Western blots, they are key in helping antibodies drive an effective immune response against a pathogen. The Fc domain creates a link between the antibody and other parts of the immune system, including the innate immune response, by binding to Fc receptors on other immune cells. (psst! Need a quick review of the different immune responses? We got you! You can also review antibodies in our Antibodies 101 animation.)
Many types of immune cells, from mast cells to B cells, express Fc receptors that bind to the Fc domain of an antigen-bound antibody and induce (or, in the case of the Fc receptors FcγRIIB1 and FcγRIIB, inhibit) specific immune responses. These receptors are specific to isotype class or even subclass, and are part of the reason why different isotypes drive different types of immune responses. Handily, the receptors are named after the isotypes they interact with, so IgG isotypes bind to Fcγ receptors; IgE isotypes bind to Fcε receptors; and so on and so forth. There are a lot of different receptors, but their downstream effects can be sorted into three main effector functions.
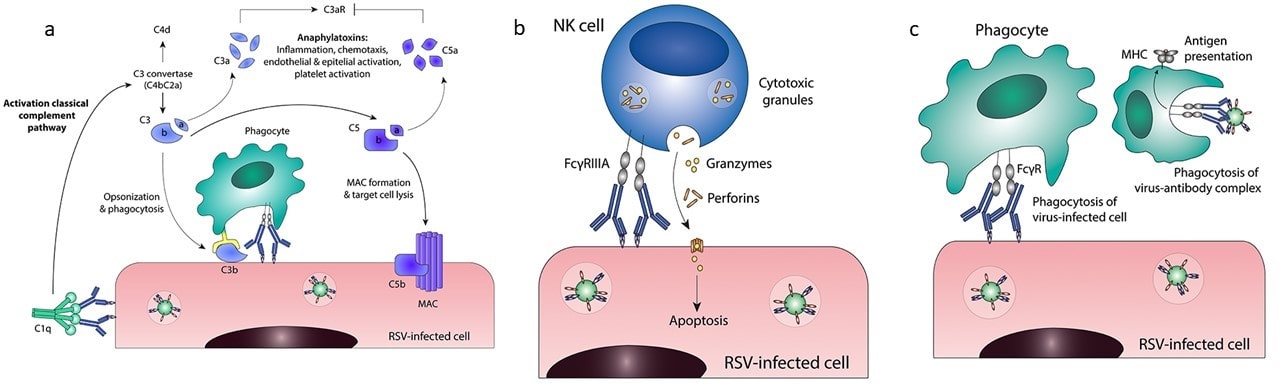 |
| Figure 1: Fc effector functions in an immune response to a viral infection a) antibody-dependent complement deposition (ADCD) b) antibody-dependent cellular cytotoxicity (ADCC) c) antibody-dependent cellular phagocytosis (ADCP). Figure adapted from van Erp, et al., 2019. |
Antibody-dependent complement deposition (ADCD)
Antibodies can bind to a pathogen and physically prevent it from entering a cell, a non-Fc-dependent effector function known as neutralization. But they can also bind to a pathogen and mark it as a target for other immune cells, in a process known as opsonization. In antibody-dependent complement deposition (ADCD), opsonization of IgG and IgM antibodies on a pathogen activates the immune system's classical complement pathway, starting a cascade of events that results in the pores being opened in the pathogen’s cell membrane (Dunkelberger & Song, 2010). As cells cannot survive when holes are punched in their membranes, the pore'd pathogens quickly lyse and die.
Antibody dependent cellular cytotoxicity (ADCC)
Antibody dependent cellular cytotoxicity (ADCC) is a slightly more direct method than opsonization. In ADCC, IgG antibodies bind to a pathogen and natural killer (NK) cells expressing Fcγ receptors binds to IgG and release cytotoxins that kill the target cell. If the pathogen is a large parasite, ADCC can be induced through a very similar interaction between IgE antibodies and eosinophils, where the eosinophils release cytotoxins via degranulation.
Antibody-dependent cellular phagocytosis (ADCP)
So far, our antibodies have induced “stabbing” and “poisoning,” but let’s be honest, the elimination style the immune system is most famous for is eating. In antibody-dependent cellular phagocytosis (ADCP), Fcγ receptors on macrophages bind IgG antibodies. The macrophages then engulf and digest the pathogen the antibodies are bound to in a process known as phagocytosis.
Other functions
We’re still learning about Fc effector functions and all that antibodies can do! For instance, some Fc receptors, once bound to an antibody, can act as immune modulators, shaping the immune response by driving particular cells or responses. And there are still antibody isotypes and Fc receptors that we don’t know much about yet. Who knows what kinds of functions they may induce?
Fc receptors are intriguing from a therapeutic standpoint. Engineered monoclonal antibodies that can drive specific Fc effector functions may be useful for inducing immunity or treating diseases (Cao, et al, 2022). So the next time you see an antibody description with the Fc domain as only providing structure and isotype determination, remember that it can do so much more than that!
References and resources
References
Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000 Apr;6(4):443-6. doi: 10.1038/74704. PMID: 10742152.
Dunkelberger, J., Song, WC. Complement and its role in innate and adaptive immune responses. Cell Res 20, 34–50 (2010). https://doi.org/10.1038/cr.2009.139
Janda, A., Bowen, A., Greenspan, N. S., & Casadevall, A. (2016). Ig Constant Region Effects on Variable Region Structure and Function. Frontiers in Microbiology, 7. https://www.frontiersin.org/articles/10.3389/fmicb.2016.00022
Torres, M., & Casadevall, A. (2008). The immunoglobulin constant region contributes to affinity and specificity. Trends in Immunology, 29(2), 91–97. https://doi.org/10.1016/j.it.2007.11.004
Xu Cao, et al. Promoting antibody-dependent cellular phagocytosis for effective macrophage-based cancer immunotherapy.Sci. Adv.8,eabl9171(2022).DOI:10.1126/sciadv.abl9171
More resources on the Addgene blog
Antibodies 101: Introduction to Antibodies
Antibodies 101: Isotypes
Antibodies 101: Chimeric Antibodies
Topics: Antibodies






Leave a Comment