Do you ever wonder about the origins of some of the common techniques or tools you use in the lab? Take for instance, the commonly used Myc-tag. Who first started using it in protein tagging experiments? Why Myc? When did the commonly used anti-c-Myc [9E10] antibody come into play? We’ll dive into those questions in this post as we explore… the life and times of the myc tag.
Origins of protein tags
For about as long as the disciplines have existed, cell and molecular biologists have been continuously pursuing new and better methods to peer into the mysterious, microscopic world of cells. The development of antibodies as tools allowed us to detect and capture proteins of interest to better study them in vivo and in vitro, especially in the mid-late 20th century with the advent of hybridoma technology and monoclonal antibodies. However, it was (and still is) infeasible to generate highly specific antibodies for every potential new target and basically impossible if you wanted to distinguish between endogenous and exogenous versions of the same target. In the 1980s, Munro and Pelham set out to address this issue by developing generic strategies for detecting cloned genes (Munro and Pelham, 1984). The strategy they landed on was to recombinantly append their proteins of interest with unrelated peptides that already had high quality antibodies available - aka peptide tagging.
Key to this strategy was the existence of high quality antibodies for well defined peptides. Munro and Pelham’s first published peptide tagging experiment involved a peptide from the neuropeptide substance P. This protein had a highly specific antibody to the C-terminal five amino acids, meaning the tag could be quite small. However, substance P has an amide group added to its C-terminus post-translationally, which turned out to be critical for antibody recognition. As such, the peptide could only be added to the C-terminus of proteins of interest and proteins tagged with it needed to be chemically treated before the antibody could detect them (Munro and Pelham, 1984). In their paper they noted that this step “...proved to be quite easy to achieve…” - but I think we can all agree that it sounds less than ideal.
Unexpected connections
Right around the same time that Munro and Pelham were devising their tagging strategy, another group of researchers was working on isolating monoclonal antibodies to better probe the functions of the human c-Myc gene (Evan, et al. 1985). Myc was one of the earliest identified oncogenes and was a target of intense research. Evan, et al. hoped that their new monoclonals would help the field explore Myc’s role in cell proliferation and tumorigenesis.
Using two different synthetic peptides corresponding to linear sequences within the middle of the human c-Myc protein or the C-terminus, Evan, et al. isolated six different monoclonal antibodies (Evan, et al. 1985). Three bound to the mid-protein peptide, while three bound to the C-terminal peptide. One of these C-terminal antibodies was Myc1-9E10 (aka anti-c-Myc [9E10]). They noticed that Myc1-9E10 and the other C-terminal antibodies only recognized the human c-Myc protein and did not recognize the mouse or chicken homologs. By comparing the homologous sequences in these species, they found that the N-terminal half of the antigen differs significantly between human and the other species, suggesting that these antibodies recognize an epitope within that region.
You’ll notice that “generate a reliable antibody for protein tagging approaches” was not part of the goal when Evan’s team generated their Myc antibodies. At first, anti-c-Myc [9E10] was just one of several new tools for unraveling the multifaceted role Myc plays in human health and disease. But no science happens in a vacuum!
Munro and Pelham learned of these new antibodies and identified Myc1-9E10 and its target peptide as a suitable pair for protein tagging. In two studies investigating mechanisms of endoplasmic reticulum localization, Munro and Pelham casually introduced the Myc-tag (Munro, et al. 1986; Munro and Pelham, 1987). They narrowed down the epitope recognized by Myc1-9E10 down to 10 amino acids (EQKLISEEDL) - larger than the substance P peptide, but still quite small. However, unlike the substance P tag, they found that the Myc epitope could be recognized by its antibody without additional chemical modification steps and when the epitope was within the tagged protein rather than at the C-terminus. These features made this tag easier to use and more flexible - it could now be used in way more applications.
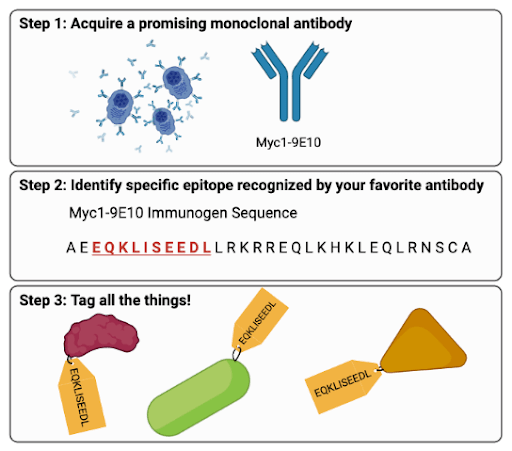 |
|
Fig. 1 Making a Protein Tag (Abridged) (1) Locate a promising monoclonal antibody with high specificity. (2) Narrow down the specific epitope that your antibody binds. For example, Myc1-9E10 binds EQKLISEEDL (pronounced “equi-kli-seed-le”). (3) Tag all the things! |
Myc1-9E10 and the Myc-tag today
Since Munro and Pelham introduced the Myc tag to the world, it has become one of the most commonly used tags in molecular biology. And the Myc1-9E10 antibody remains a trusty antibody partner; as I write this, there are close to 10,000 citations for anti-Myc 9E10 in CiteAb, many of which are from this year.
But are we all really using the same antibody developed almost 40 years ago? Well…kinda. In some cases, antibody providers are distributing monoclonal antibodies derived from the same hybridoma developed by Evans, et al. In other cases, providers have moved to producing anti-Myc [9E10] as a recombinant antibody. Researchers first sequenced the variable domains of Myc1-9E10 in the late 1990s (Fuchs, et al. 1997; Schiweck, et al. 1997) with the goal of making the antibody more amenable to protein engineering. As a result, you can now find Myc1-9E10 in a variety of isotypes like rat IgG1, human IgG2, mouse IgE, goat IgG, (and more!) as well as the original mouse IgG1. And the refinement of this trusty tool doesn’t end there: you can also find anti-Myc [9E10] Fab fragments and single-chain variable fragments.
 |
| Abi, on the other hand, might just be the antibody Mary Poppins! |
All of the examples of new versions of Myc1-9E10 mentioned above basically involve taking the Myc1-9E10 variable regions and sticking them onto different scaffolds. But over the years, several alternative antibodies against the Myc tag have been generated in an attempt to overcome some of Myc1-9E10’s shortcomings. Wait, 9E10 isn’t the Mary Poppins of antibodies (absolutely perfect in every way)? Unfortunately, no antibody is that perfect. One of the known limitations was described by Schüchner, et al., who published a paper showing that the amino acids neighboring the Myc epitope influence 9E10’s affinity for the epitope, a phenomenon known as sequence context sensitivity (Schüchner, et al. 2020). In their study, some of the newer Myc-tag antibodies appeared to be less sensitive to sequence context.
So, do Schüchner, et al.’s results mean the end of 9E10’s time in the saga of the Myc tag? Hardly! 9E10’s accessibility and the variety of formats you can find it in likely mean it will remain an important tool for the foreseeable future. These newer findings simply add to our understanding of how this antibody works and allow users to better troubleshoot and design experiments. It turns out that even with the most popular antibodies, context is key.
References and Resources
References
Evan GI, Lewis GK, Ramsay G, Bishop JM (1985) Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol 5:3610–3616. https://doi.org/10.1128/mcb.5.12.3610-3616.1985
Munro S, Pelham HR (1984) Use of peptide tagging to detect proteins expressed from cloned genes: deletion mapping functional domains of Drosophila hsp 70. Embo J 3:3087–3093. https://doi.org/10.1002/j.1460-2075.1984.tb02263.x
Munro S, Pelham HRB (1986) An hsp70-like protein in the ER: Identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell 46:291–300. https://doi.org/10.1016/0092-8674(86)90746-4
Munro S, Pelham HRB (1987) A C-terminal signal prevents secretion of luminal ER proteins. Cell 48:899–907. https://doi.org/10.1016/0092-8674(87)90086-9
Schüchner S, Behm C, Mudrak I, Ogris E (2020) The Myc tag monoclonal antibody 9E10 displays highly variable epitope recognition dependent on neighboring sequence context. Sci Signal 13. https://doi.org/10.1126/scisignal.aax9730
Additional resources on the Addgene blog
- Antibodies 101: Monoclonal Antibodies
- Plasmids 101: Peptide Tagging
- Plasmid-based Recombinant Antibody
Resources on Addgene.org
- Find recombinant anti-c-Myc [9E10] at Addgene
- Find plasmids with the myc-tag
Topics: Antibodies






Leave a Comment