While the antibodies present throughout our bodies carry out plenty of roles just the way they are, the research antibodies in your refrigerator often need a little help to be useful. Mainly because, well, antibodies are kind of hard to see. To solve this issue, researchers conjugate various labels to antibodies that produce detectable signals like light or color. In this post, we’ll go over some of the common conjugates you may encounter and how conjugates and antibodies come together.
Common conjugates in the lab
Fluorophores
Fluorophores allow you to detect your antibodies using techniques like fluorescence microscopy, flow cytometry, or spectrophotometry. The range of fluorophores available for antibody conjugation spans the visible spectrum, so they are great for multiplexing and can be used in a variety of assay types. Be sure to check the spectral properties of the fluorophores you are considering to make sure that they’ll be compatible with your instruments, each other, and your target. I love FPbase’s Spectra Viewer tool for comparing different fluorophores. We also touched on fluorophores as antibody conjugates in an earlier post, where you can find some additional tips for working with these tools.
Enzymes
An equally, if not more, common conjugate class than fluorophores are enzymes. The most common enzyme conjugates are Horseradish Peroxidase (HRP) and Alkaline Phosphatase (AP). Various substrates for each of these enzymes allow you to detect enzyme activity (and therefore your antibody) either through a chromogenic or chemiluminescent reaction. Chemiluminescence, which is light produced by a chemical reaction, can be detected using specialized imaging instruments or X-ray film and lends itself well to assays like western blots and ELISAs. Chromogenic reactions produce a color change, so they are typically used in microscopy-based assays like immunohistochemistry, though they are also a common choice for ELISAs.
Biotin
Biotin is used in a range of antibody-based assays. Biotin itself is not visible or capable of producing a visible signal, but it has a strong affinity for avidin and streptavidin, both of which can bind up to four biotin molecules and can themselves be linked to a labeling molecule. These properties allow for efficient signal amplification, making biotin conjugated antibodies good options for detecting low abundance targets. Check out our post on Biotinylation to learn more about biotin and its use in molecular biology.
Other Conjugates to Know
It would be overwhelming to try to cover all of the possible antibody conjugates (especially if you start to consider clinical applications). But there are a few other classes that we would be remiss not to at least mention. Oligonucleotides allow for sensitive detection of low abundance targets with significant multiplexing potential. You will find antibody-oligonucleotide conjugates used in assays like proximity ligation, immuno-PCR, and single-cell applications (Hegazy, et al., 2020; Niemeyer, et al., 2007; Stoeckius, et al., 2017). Metal isotopes are useful for mass cytometry (Bendall, et al., 2012). And, you can also conjugate antibodies directly to beads for purification purposes.
Coupling up - antibody conjugation chemistry
Whatever the conjugate, the molecules must somehow be tightly linked to your antibody. This chemical coupling can be achieved through several different mechanisms. Most take advantage of chemical groups intrinsically present on antibodies, such as the primary amine groups from lysines, sulfhydryl groups from cysteines, or carbohydrates from glycosylation (Dennler, et al., 2015). However, there are certainly other approaches that involve modifying the antibody sequence or that rely on strong protein-protein interactions with other peptides, like an Fc-binding domain (Figure 1).
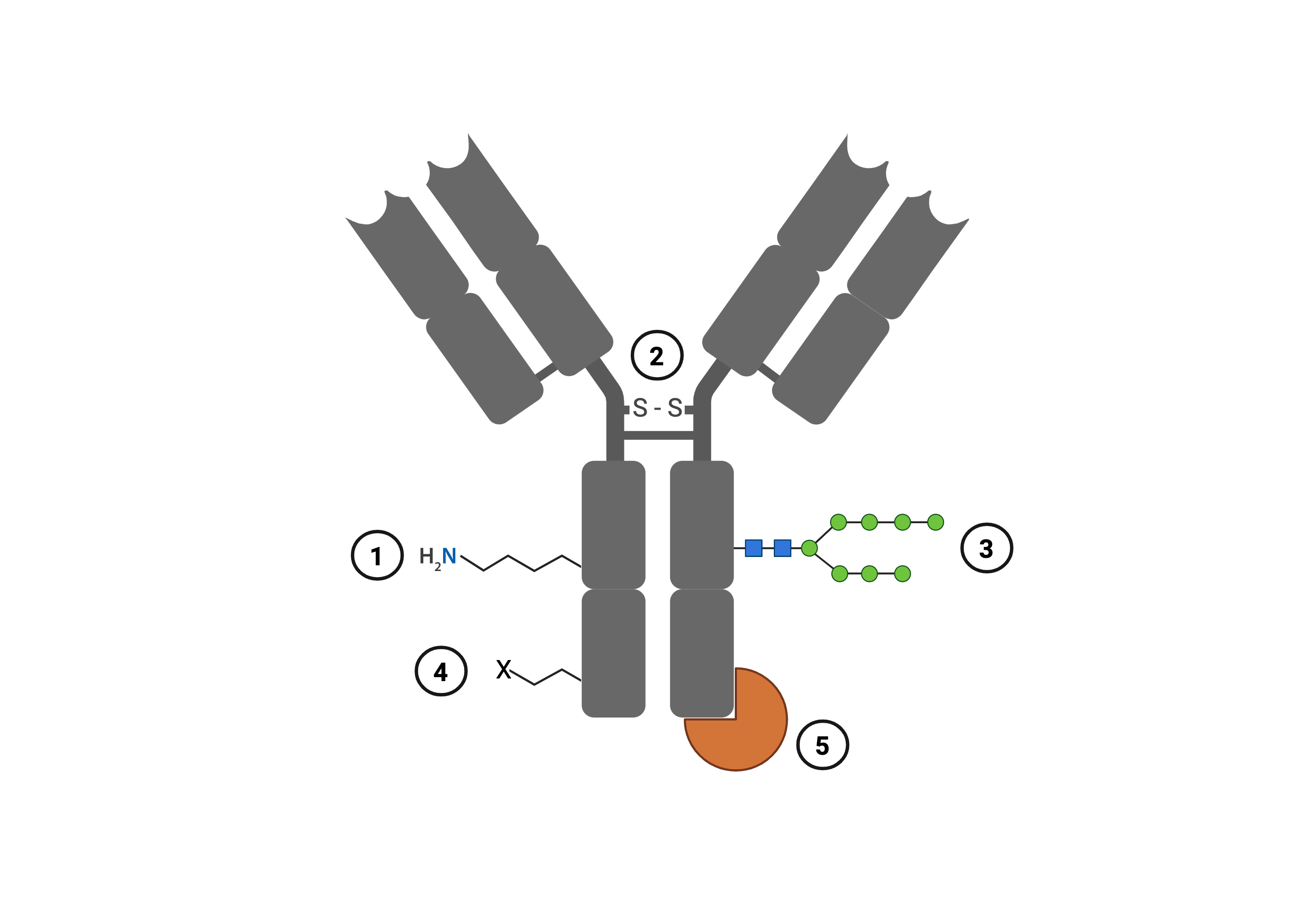 |
| Figure 1: Examples of sites on antibodies used for conjugation. 1) Endogenous lysine residues, which can be found throughout the antibody. 2) Endogenous cysteines, such as those that make up the disulfide bonds between antibody chains. 3) Endogenous carbohydrates added to antibodies post-translationally. 4) Modifications made to antibodies, such as inclusion of non-canonical amino acids or peptide tags. 5) Strong protein-protein interactions with other peptides. Created with BioRender.com. |
Many biologists can lead perfectly blissful, productive lives without thinking about these chemical reactions. After all, there is an abundance of commercially available conjugated antibodies that are ready to use off the shelf. So why are we talking about this? Even though there are so many commercially conjugated antibodies available, that never guarantees the antibody you need will be conjugated, or that it will be conjugated to the conjugate of your choice. Knowing you can conjugate an antibody yourself allows you to consider a broader selection of antibodies for your experiments.
Many of the methods for antibody conjugation can be quite variable from antibody to antibody or batch to batch. For example, take lysine conjugation, which is commonly used for labeling research antibodies. Lysine residues are found throughout antibody sequences, up to 80 per antibody (Mueller, et al., 1988). If some of those lysines are in the antigen binding region or even just nearby, then conjugation could impact the antibody’s antigen recognition and affinity. Hopefully, a commercially available conjugated antibody will have been verified to perform the same as its unconjugated version, but if you find yourself doing your own conjugation, it is important to keep in mind that you will need to confirm your antibodies’ performance after the reaction.
If you are looking to conjugate antibodies yourself, there are kits available to make the process quick and easy. Before getting started, be sure to check that your antibody’s buffer is compatible with your conjugation kit, since many common antibody buffers and buffer additives can impair the reaction or the functionality of the conjugate. For example, Addgene’s antibodies are provided in a buffer with the antimicrobial agent sodium azide, which can interfere with conjugation reactions. If needed, you can often remove the incompatible agents by dialysis or gel filtration.
There is, of course, a lot more to explore in the world of antibody conjugation. But hopefully, this introduction provides a good base from which to dive deeper!References and resources
References
Bendall SC, Nolan GP, Roederer M, Chattopadhyay PK (2012) A deep profiler’s guide to cytometry. Trends Immunol 33:323–332. https://doi.org/10.1016/j.it.2012.02.010
Dennler P, Fischer E, Schibli R (2015) Antibody Conjugates: From Heterogeneous Populations to Defined Reagents. Antibodies 4:197–224. https://doi.org/10.3390/antib4030197
Hegazy M, Cohen-Barak E, Koetsier JL, et al (2020) Proximity Ligation Assay for Detecting Protein-Protein Interactions and Protein Modifications in Cells and Tissues in Situ. Curr Protoc Cell Biol 89:e115. https://doi.org/10.1002/cpcb.115
Mueller BM, WrasidloO WA, Reisfeld RA (1988) Determination of the Number of e-Amino Groups Available for Conjugation of Effector Molecules to Monoclonal Antibodies. Hybridoma 7:453–456. https://doi.org/10.1089/hyb.1988.7.453
Niemeyer CM, Adler M, Wacker R (2007) Detecting antigens by quantitative immuno-PCR. Nat Protoc 2:1918–1930. https://doi.org/10.1038/nprot.2007.267
Stoeckius M, Hafemeister C, Stephenson W, et al (2017) Simultaneous epitope and transcriptome measurement in single cells. Nat Methods 14:865–868. https://doi.org/10.1038/nmeth.4380
Additional resources on the Addgene blog
Antibodies 101: Buffers, Storage, and Conjugates
Antibodies 101: The Basics of Western Blotting
Plasmids 101: Biotinylation
Resources on Addgene.org
Unconjugated antibodies at AddgeneAntibody plasmids at Addgene
Topics: Antibodies, antibodies 101







Leave a Comment