 It was by serendipity that I got into the field of gene therapy, more specifically AAV-based retinal gene therapy. The year was 2001 and I started a job as a technician in a lab using adeno-associated viral vectors (AAVs) to treat an inherited retinal degenerative disease called Retinitis Pigmentosa. I quickly became fascinated by this emerging technology and its potential for the treatment of some genetic diseases.
It was by serendipity that I got into the field of gene therapy, more specifically AAV-based retinal gene therapy. The year was 2001 and I started a job as a technician in a lab using adeno-associated viral vectors (AAVs) to treat an inherited retinal degenerative disease called Retinitis Pigmentosa. I quickly became fascinated by this emerging technology and its potential for the treatment of some genetic diseases.
I didn’t know it at the time, but gene therapy had fallen - plummeted really - from grace 2 years prior with the death of Jesse Gelsinger in an adenovirus-based gene therapy clinical trial gone horribly wrong. It brought the whole field to a grinding stop and only now, 2 decades later, is it finally back in the limelight. On December 19, 2017 the FDA approved Luxturna™ the first gene therapy for the treatment of a rare genetic eye disease, Leber’s congenital amaurosis. This is the first of what I, scientists, doctors and patients hope are many more clinical successes for gene (and cell) therapies.
One may wonder why the first AAV-based gene therapy treatment for a genetic disease approved by the FDA is for a rare eye disease and not another genetic disease more prevalent or life-threatening (cystic fibrosis, hemophilia, muscular dystrophy)?
AAV gene therapy started in the mid-90's with a lot of work and a little bit of luck
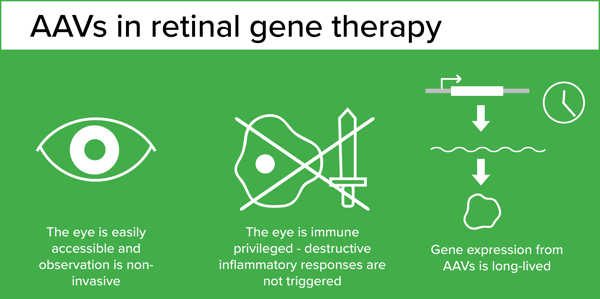
A pioneer in the field, Dr. Jean Bennett started working on ways to deliver genes to correct genetic conditions over 25 years ago when completing her PhD and medical training in Ophthalmology. Gene therapy was in its infancy, but the eye was identified early on as an ideal target. It is an easily accessible organ that can be monitored non-invasively over time. Importantly, it is an immune privileged compartment, meaning that it can tolerate the presence of antigens (such as viral vectors) without triggering a destructive inflammatory immune response. At the time, adenoviral vectors were the most commonly used viral vectors for gene delivery, and they had been shown to to transduce the retina but only transiently. However new viral vectors capable of providing long-term gene expression in retinal cells - AAV - were becoming available.
At the same time advances in human genetics led to the identification of several disease-causing genes, including the RPE65 gene responsible for an autosomal recessive, severe, childhood-onset form of blindness name Leber’s Congenital Amaurosis (LCA). Shortly after the cloning of the RPE65 gene, a naturally occurring animal model, the RPE65−/− dog, was characterized and found to suffer from early and severe visual impairment that faithfully recapitulated the human condition.
Luck brought all these key elements together at the right time and place. But it is the hard work and persistence of a dedicated team of scientists led by Dr. Jean Bennett (UPenn) that resulted in the groundbreaking 2001 study showing that AAV-mediated delivery of the RPE65 gene restores vision in the dog model of LCA (Lancelot, one of the dogs who received the AAV-RPE65 treatment, famously visited Congress to help increase awareness about the potential of gene therapy!). It would take another 6 years before the 1st clinical trial started, and 10 more years to obtain FDA approval and make history in the form of Luxturna™.
A better vector for ocular gene therapy: Adeno-Associated Virus (AAV)
While other viral vectors such as adenovirus (AdV) and lentivirus (LV) are known to transduce retinal cells, AAV quickly became the vector of choice for ocular gene therapy.
AdV and LV have been used successfully in preclinical and clinical settings for ocular conditions. However, AdV was abandoned early on due to its considerable immunogenicity and its transient expression. LV results in long term expression in dividing and non-dividing cells due to its (random) integration into the genome, and its large DNA packaging capacity is well suited for larger transgenes. Unfortunately it has limited tropism in the retina, primarily infecting cells of the retinal pigmented epithelium (RPE).
AAVs, on the other hand, have broad tropism in the eye. AAV-2, the first identified serotype and the one used in Luxturna™, can efficiently transduce various types of retinal neurons. Nowadays a toolbox of naturally occurring and engineered AAV serotypes that further expands the range of AAV properties is available. While only natural serotypes are currently used in clinical trials, a variety of engineered ones have shown great promise in preclinical studies and could reach clinical trials soon.
The AAV safety profile is excellent and further strengthens its position as the vector of choice for in vivo gene therapy. It is not known to cause any disease in humans, and its low immunogenicity is critical when systemic delivery of high doses of vectors are needed. Additionally, AAVs do not integrate in the host genome but still lead to long-term (>10 years) transgene expression in non-dividing cells because they remain in episomal form in the cell’s nucleus.
But since nothing’s ever perfect, neither is AAV. Its main limitation is the small size of its DNA cargo - 4.8 kb at most - which means that a gene larger than 3 kb cannot be delivered using this vector. For example, although researchers know that Usher Syndrome (a disease that results in combined deafness and blindness) is caused by defects in the gene MYO7a, at ~7 kb this gene is too large to deliver via AAV. Dual vectors strategies are being optimized to overcome this limitation, with limited success so far. Luckily for patients, this gene can be targeted to RPE cells using a lentiviral vector and a clinical trial is ongoing.
Moving forward with ocular gene therapy
The pipeline of AAV-based ocular gene therapies is impressive: clinical trials for choroideremia, X-linked retinitis pigmentosa, achromatopsia, and age-related macular degeneration are ongoing and some will hopefully follow in the footsteps of Luxturna™. However, despite promising results in preclinical studies some potential candidates for rare to ultra-rare ocular diseases are being benched due to the high cost of clinical development. As a result, Luk Vandenberghe and colleagues at MEEI co-founded a non-profit to help push forward candidate therapies for these diseases.
Riding on the early successes of retinal gene therapy, an ever-growing number of clinical trials for devastating genetic diseases such as hemophilia, muscular dystrophy, spinal muscular atrophy, neurodegenerative diseases and metabolic diseases are also underway and reported results are encouraging.
Importantly, a significant amount of basic research is being done to further improve the technology: engineering of new capsid variants, designing expression cassettes to regulate and optimize transgene levels, and large scale manufacturing of viral vectors to support trials for prevalent diseases - to name a few.
CRISPR-based tools are also particularly well suited to be combined with AAV for in vivo gene editing, and reports suggest that the first clinical study combining these tools may start as early as next year.
Twenty years after falling from grace, gene therapy is back, better and safer. It is now one of the most active and exciting areas of science. This is hopefully only the beginning of a gene therapy revolution and the development of personalized medicine.
References and further reading
1. FDA approves gene therapy for blindness
2. Gene Therapy emerges from disgrace to be the next big thing, again
3. Acland G. et al., 2001. Gene therapy restores vision in a canine model of childhood blindness. Nature Genetics 28, 92-95. PubMed PMID: 11326284.
4. Bennett J, 2017. Taking Stock of Retinal Gene Therapy: Looking Back and Moving Forward. Molecular Therapy Vol. 25 No 5. PubMed PMID: 28391961. PubMed Central PMCID: PMC5417792.
5. Naldini,L., 2015. Gene Therapy returns to centre stage. Nature Vol 526, p.351. PubMed PMID: 26469046.
6. The Forever Fix: Gene Therapy and the Boy Who Saved It by Ricki Lewis. ISBN-13: 978-1250015778
7. Yu W and Wu Z., 2018. In vivo Applications of CRISPR-Based Genome Editing in the Retina. Front Cell Dev Biol. 2018 May 14;6:53. Review. PubMed PMID: 29868583. PubMed Central PMCID: PMC5960719.
8. Trapani I and Auricchio A., 2018. Seeing the Light after 25 Years of Retinal Gene Therapy. Trends Mol Med. 2018, S1471-4914(18)30123-0. DOI: 10.1016/j.molmed.2018.06.006. PubMed PMID: 29983335.
9. Day TP1, Byrne LC, Schaffer DV, Flannery JG, 2014. Advances in AAV vector development for gene therapy in the retina. Adv Exp Med Biol. 2014; 801: 687–693. doi: 10.1007/978-1-4614-3209-8_86. PubMed PMID: 24664759. PubMed Central PMCID: PMC4267879.
10. Samulski RJ, Muzyczka N., 2014. AAV-Mediated Gene Therapy for Research and Therapeutic Purposes. Annu Rev Virol. 2014 Nov;1(1):427-51. DOI: 10.1146/annurev-virology-031413-085355. PubMed PMID: 26958729.
Additional Resources on the Addgene Blog
- Adeno-Associated Virus (AAV) for cell and gene therapy
- AAVs for genome editing
- AAV: A versatile tool for gene expression in mammals
Resources at Addgene.org
- Find AAV plasmids
- Find ready-to-use AAV viral preps
- Browse all viral vectors
Topics: Viral Vectors, AAV







Leave a Comment