This post was contributed by Kusumika (Kushi) Mukherjee.
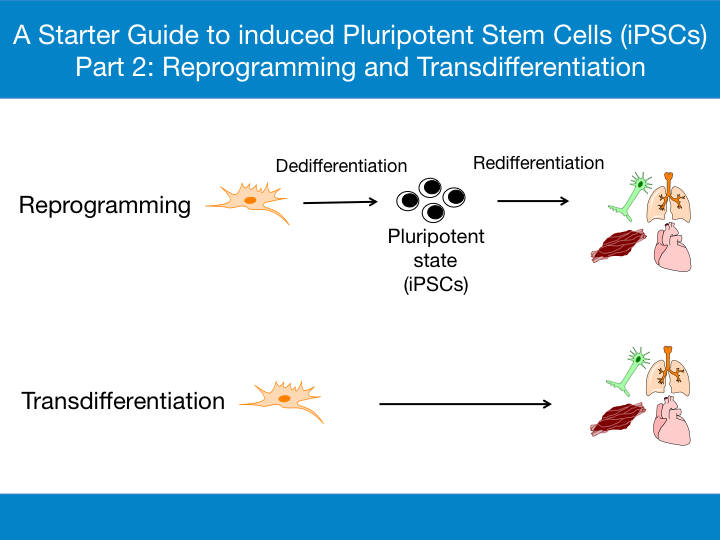
The ultimate goal in the field of regenerative medicine is to replace lost or damaged cells. Here, I will discuss the two major processes by which an adult somatic cell is converted to a different cell type for regeneration and repair and situations where one process is favored over the other.
Cell conversion happens via:
- Reprogramming
A differentiated somatic cell reverts back to a pluripotent state, which then proliferates and is redifferentiated to a different cell type. - Transdifferentiation
In this process, differentiated adult somatic cells are converted to cells of a different lineage, without dedifferentiating into a pluripotent stage.
Cellular reprogramming
The reversal of a differentiated cell type to an undifferentiated state and then redifferentiation into the cell type of choice in vitro is known as reprogramming [1]. The process can be divided into two stages:
- Dedifferentiation - Conversion of adult somatic cells into the pluripotent state.
- Redifferentiation - Conversion of the pluripotent cells into differentiated cells of choice.
The dedifferentiation stage involves overexpression of four reprogramming factors- OCT4, SOX2, KLF4, and C-MYC - that induce a differentiated somatic cell to revert back to a pluripotent stage (iPSC formation) [2, 3]. The iPSCs then proliferate and redifferentiate to another cell type of choice. The four reprogramming factors can be delivered and expressed in multiple somatic cells via various methods. Some of the more common delivery methods include retrovirus [2], lentivirus [4], adenovirus [5], Sendai virus [6], plasmid electroporation (episomal) [7, 8] and mRNA transfection [9]. Many of the plasmids used for these methods can be found on Addgene’s stem cell page. On this page, you can also find a table with a list of methods and the species they were used in. iPSCs have now been generated from many different types of somatic cells. The goal is to use cells that can be easily isolated from donors. Apart from fibroblasts, human keratinocytes from hair pluck, peripheral blood cells, and renal epithelial cells from urine are some of the easily isolated somatic cells that have been reprogrammed to iPSCs successfully [10-12].
The next stage of reprogramming consists of redifferentiation of iPSCs into the cell type of choice. This step is sometimes also referred to as “directed differentiation.” Specific cell media, supplements, bioactive small molecules, and growth factors are used to control the cell fate of iPSCs and differentiate them into different cell lineages [13]. Over the last decade, many cell types have been successfully differentiated from human iPSCs. Below is a list of some of these cell types [13]:
| Germ cells [14] | Hepatocytes [15] |
| Pancreatic β-cells [16] | Intestinal tissue [17] |
| Lung epithelial cells [18] | Red blood cells [19, 20] |
| Osteoclasts and osteoblasts [21, 22] | Cardiomyocytes [23, 24] |
| Smooth muscle cells [25] | Skeletal myogenic cells [26] |
| Chondrocytes [27] | Adipocytes [28] |
| Keratinocytes [29] | Photoreceptors [30] |
| Otic hair cells [31] | Neurons [32, 33, 34] |
You can find a variety of plasmids for differentiation here.
Transdifferentiation
Dedifferentiation to an intermediate pluripotent state is not always obligatory in cell conversion processes [35]. Rather than reprogram cells all the way back to their most primitive pluripotent stem cell state, through transdifferentiation adult somatic cells are converted directly into a different cell type, bypassing the lengthy processes of reprogramming. The process was first observed in the regenerating lens of the newt over 100 years ago [36]. While natural transdifferentiation is rare in mammals, an example is observed in the pancreas when excess β-cell damage results in the transdifferentiation of glucagon-producing α-cells into insulin-producing β-like-cells [37, 38].
In 1987, Davis et al. reported one of the earliest examples of transdifferentiation in vitro where treatment of mouse fibroblasts with 5-azacytidine led to their conversion into myoblasts [39]. In 2000, Ferber et al. showed for the first time that mouse liver cells could be transdifferentiated in vivo to pancreatic β-like-cells with the expression of pancreatic and duodenal homoeobox gene1 (PDX1) [40]. In recent works, transdifferentiation is usually carried out by expressing transcription factors specific to the lineage of the target cell in the original somatic cells [41]. The in vivo and in vitro methods are similar except that the vectors carrying the transdifferentiation factors are directly injected into the organ of interest for in vivo transdifferentiation. Multiple cell types such as fibroblasts, hepatocytes, and pancreatic exocrine cells have been successfully transdifferentiated into neurons and β-cells [40-42].
Reprogramming and transdifferentiation: When to use one over the other
Both reprogramming and transdifferentiation convert differentiated somatic cells into another cell type. However, these two approaches differ in several ways. Below is a table listing some of critical differences (adapted from Zhou and Melton, 2008, [43]):
| Characteristic | Reprogramming+ | Transdifferentiation |
| in vivo or in vitro | in vitro | Both in vitro and in vivo |
| Epigenetic marks | Complete removal to convert to iPSCs and re-establishment after | Partial rearrangement from one cell type to another |
| Time and resources | More (due to additional pluripotency stage) | Less |
| Safety | Less (C-MYC, a known oncogene is one of the reprogramming factors) | More |
| Chances of accumulation of harmful mutations | More, due to selection from more cycles of proliferation | Less |
| Cell types | All types | Less |
| Ease of genetic correction | Easy | Difficult |
Overall, reprogramming is very flexible. It offers unlimited potential to produce all cell types in the body. On the other hand, only few cell types have been currently transdifferentiated successfully, limiting the utility of this process. Moreover, it is much easier to genetically modify cells during the reprogramming process as they are propagated in vitro as part of the process. This opens up a wide range of possibilities in clinical situations. In cases where the objective is to fix a disease-inducing genetic mutation in a patient, trying to transdifferentiate any of the patient’s cells will not alleviate the problem. The best option then would be to dedifferentiate cells from the patient in vitro then correct the damaged gene in the resulting iPSCs before differentiating the cells into the correct lineage and returning them back to the patient.
In this post, I have detailed the two major processes by which cells are converted to replenish and repair cells that are lost or damaged. Both transdifferentiation and reprogramming give researchers the ability to convert a differentiated cell to a different cell type. While transdifferentiation is suited for switching cell types between similar lineages, reprogramming is more versatile and universal.
Many thanks to our guest blogger, Kusumika (Kushi) Mukherjee.
 Kusumika (Kushi) Mukherjee is the Editor of Trends in Pharmacological Sciences, a Cell Press reviews journal. She joined Cell Press to pursue a career in science communication and publishing after completing her postdoctoral training from Massachusetts General Hospital and Harvard Medical School. Connect with her on LinkedIn @ https://www.linkedin.com/in/
Kusumika (Kushi) Mukherjee is the Editor of Trends in Pharmacological Sciences, a Cell Press reviews journal. She joined Cell Press to pursue a career in science communication and publishing after completing her postdoctoral training from Massachusetts General Hospital and Harvard Medical School. Connect with her on LinkedIn @ https://www.linkedin.com/in/
References
1. Hochedlinger, K. and R. Jaenisch, Nuclear reprogramming and pluripotency. Nature, 2006. 441(7097): p. 1061-7. PubMed PMID: 16810240.
2. Takahashi, K., et al., Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 2007. 131(5): p. 861-72. PubMed PMID: 18035408.
3. Takahashi, K. and S. Yamanaka, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 2006. 126(4): p. 663-76. PubMed PMID: 16904174.
4. Yu, J., et al., Induced pluripotent stem cell lines derived from human somatic cells. Science, 2007. 318(5858): p. 1917-20. PubMed PMID: 18029452.
5. Stadtfeld, M., et al., Induced pluripotent stem cells generated without viral integration. Science, 2008. 322(5903): p. 945-9. PubMed PMID: 18818365. PubMed Central PMCID: PMC3987909.
6. Ban, H., et al., Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc Natl Acad Sci U S A, 2011. 108(34): p. 14234-9. PubMed PMID: 21821793. PubMed Central PMCID: PMC3161531.
7. Yu, J., et al., Human induced pluripotent stem cells free of vector and transgene sequences. Science, 2009. 324(5928): p. 797-801. PubMed PMID: 19325077. PubMed Central PMCID: PMC2758053.
8. Okita, K., et al., Generation of mouse induced pluripotent stem cells without viral vectors. Science, 2008. 322(5903): p. 949-53. PubMed PMID: 18845712.
9. Warren, L., et al., Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell, 2010. 7(5): p. 618-30. PubMed PMID: 20888316. PubMed Central PMCID: PMC3656821.
10. Aasen, T., et al., Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol, 2008. 26(11): p. 1276-84. PubMed PMID: 18931654.
11. Loh, Y.H., et al., Reprogramming of T cells from human peripheral blood. Cell Stem Cell, 2010. 7(1): p. 15-9. PubMed PMID: 20621044. PubMed Central PMCID: PMC2913590.
12. Zhou, T., et al., Generation of human induced pluripotent stem cells from urine samples. Nat Protoc, 2012. 7(12): p. 2080-9. PubMed PMID: 23138349.
13. Williams, L.A., B.N. Davis-Dusenbery, and K.C. Eggan, SnapShot: directed differentiation of pluripotent stem cells. Cell, 2012. 149(5): p. 1174-1174 e1. PubMed PMID: 22632979.
14. Sasaki, K., et al., Robust In Vitro Induction of Human Germ Cell Fate from Pluripotent Stem Cells. Cell Stem Cell, 2015. 17(2): p. 178-94. PubMed PMID: 26189426.
15. Si-Tayeb, K., et al., Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology, 2010. 51(1): p. 297-305. PubMed PMID: 19998274. PubMed Central PMCID: PMC2946078.
16. Zhang, D., et al., Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res, 2009. 19(4): p. 429-38. PubMed PMID: 19255591.
17. Spence, J.R., et al., Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature, 2011. 470(7332): p. 105-9. PubMed PMID: 21151107. PubMed Central PMCID: PMC3033971.
18. Huang, S.X., et al., Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol, 2014. 32(1): p. 84-91. PubMed PMID: 24291815. PubMed Central PMCID: PMC4101921.
19. Dias, J., et al., Generation of red blood cells from human induced pluripotent stem cells. Stem Cells Dev, 2011. 20(9): p. 1639-47. PubMed PMID: 21434814. PubMed Central PMCID: PMC3161101.
20. Chang, C.J., et al., Production of embryonic and fetal-like red blood cells from human induced pluripotent stem cells. PLoS One, 2011. 6(10): p. e25761. PubMed PMID: 22022444. PubMed Central PMCID: PMC3192723.
21. Grigoriadis, A.E., et al., Directed differentiation of hematopoietic precursors and functional osteoclasts from human ES and iPS cells. Blood, 2010. 115(14): p. 2769-76. PubMed PMID: 20065292. PubMed Central PMCID: PMC2854424.
22. Jeon, O.H., et al., Human iPSC-derived osteoblasts and osteoclasts together promote bone regeneration in 3D biomaterials. Sci Rep, 2016. 6: p. 26761. PubMed PMID: 20065292. PubMed Central PMCID: PMC2854424.
23. Burridge, P.W., et al., Chemically defined generation of human cardiomyocytes. Nat Methods, 2014. 11(8): p. 855-60. PubMed PMID: 24930130. PubMed Central PMCID: PMC4169698.
24. Lian, X., et al., Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A, 2012. 109(27): p. E1848-57. PubMed PMID: 22645348. PubMed Central PMCID: PMC3390875.
25. Patsch, C., et al., Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat Cell Biol, 2015. 17(8): p. 994-1003. PubMed PMID: 26214132. PubMed Central PMCID: PMC4566857.
26. Maffioletti, S.M., et al., Efficient derivation and inducible differentiation of expandable skeletal myogenic cells from human ES and patient-specific iPS cells. Nat Protoc, 2015. 10(7): p. 941-58. PubMed PMID: 26042384.
27. Nejadnik, H., et al., Improved approach for chondrogenic differentiation of human induced pluripotent stem cells. Stem Cell Rev, 2015. 11(2): p. 242-53. PubMed PMID: 25578634. PubMed Central PMCID: PMC4412587.
28. Mohsen-Kanson, T., et al., Differentiation of human induced pluripotent stem cells into brown and white adipocytes: role of Pax3. Stem Cells, 2014. 32(6): p. 1459-67. PubMed PMID: 24302443.
29. Kogut, I., D.R. Roop, and G. Bilousova, Differentiation of human induced pluripotent stem cells into a keratinocyte lineage. Methods Mol Biol, 2014. 1195: p. 1-12. PubMed PMID: 24510784. PubMed Central PMCID: PMC4096605.
30. Lamba, D.A., et al., Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One, 2010. 5(1): p. e8763. PubMed PMID: 20098701.
31. Tang, Z.H., et al., Genetic Correction of Induced Pluripotent Stem Cells From a Deaf Patient With MYO7A Mutation Results in Morphologic and Functional Recovery of the Derived Hair Cell-Like Cells. Stem Cells Transl Med, 2016. 5(5): p. 561-71. PubMed PMID: 27013738. PubMed Central PMCID: PMC4835250.
32. Ma, L., Y. Liu, and S.C. Zhang, Directed differentiation of dopamine neurons from human pluripotent stem cells. Methods Mol Biol, 2011. 767: p. 411-8. PubMed PMID: 21822892.
33. Shi, Y., P. Kirwan, and F.J. Livesey, Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc, 2012. 7(10): p. 1836-46. PubMed PMID: 22976355.
34. Wang, S., et al., Differentiation of human induced pluripotent stem cells to mature functional Purkinje neurons. Sci Rep, 2015. 5: p. 9232. PubMed PMID: 25782665. PubMed Central PMCID: PMC4363833.
35. Eguizabal, C., et al., Dedifferentiation, transdifferentiation, and reprogramming: future directions in regenerative medicine. Semin Reprod Med, 2013. 31(1): p. 82-94. PubMed PMID: 23329641.
36. Jopling, C., S. Boue, and J.C. Izpisua Belmonte, Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol, 2011. 12(2): p. 79-89. PubMed PMID: 21252997.
37. Merrell, A.J. and B.Z. Stanger, Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nat Rev Mol Cell Biol, 2016. 17(7): p. 413-25. PubMed PMID: 26979497.
38. Thorel, F., et al., Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature, 2010. 464(7292): p. 1149-54. PubMed PMID: 20364121. PubMed Central PMCID: PMC2877635.
39. Davis, R.L., H. Weintraub, and A.B. Lassar, Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell, 1987. 51(6): p. 987-1000. PubMed PMID: 3690668.
40. Ferber, S., et al., Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med, 2000. 6(5): p. 568-72. PubMed PMID: 10802714.
41. Vierbuchen, T., et al., Direct conversion of fibroblasts to functional neurons by defined factors. Nature, 2010. 463(7284): p. 1035-41. PubMed PMID: 20107439. PubMed Central PMCID: PMC2829121.
42. Zhou, Q., et al., In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature, 2008. 455(7213): p. 627-32. PubMed PMID: 18754011.
43. Zhou, Q. and D.A. Melton, Extreme makeover: converting one cell into another. Cell Stem Cell, 2008. 3(4): p. 382-8. PubMed PMID: 18940730.
Additional Resources on the Addgene Blog
- Stem Cell Models for Disease & Open Science: Interview with Darrell Kotton
- Four Factors that Differentiate the Stem Cell Field
- Learn how lentiviral vectors can be used for gene delivery
Resources on Addgene.org
- Visit our Stem Cell Pages
- Find Viral Vectors for Your Research
- Find Plasmids from the Yamanaka Lab
Topics: Stem Cells, Other






Leave a Comment