Viruses are the SUVs of molecular biology – they can deliver materials to specific destinations, haul a variety of cargo, and even handle a variety of terrains. They are, in fact, a choice delivery vector from basic science research all the way to clinical gene therapy. But how can you use these amazing tools in your experiments? Let’s unpack the myriad number of emerging viral applications and give you some recommendations for putting viruses to work.
Viral applications
Viruses work by infecting a host cell (the target of viral infection) and delivering a genetic payload. This cargo is typically their own genome, but this step can be engineered to be anything you want delivered to your target cells. This delivered DNA/RNA can then be either permanently integrated into the genome of the target cell or transiently expressed.
It’s important to note that for all the applications we discuss, there is more than one type of virus to choose from (lentivirus, retrovirus, AAV, etc.). When you’ve decided on an application, our Viruses as Biological Tools blog can help you learn about the different types of viruses and pick one for your experiment.
Research applications
Gene expression control
Researchers are always wanting to manipulate gene expression – turn it up, turn it down, knock it out, etc. It’s how we understand what genes do! Viruses have several options to allow you to do exactly that:
- shRNAs are short RNA sequences which target complementary mRNA sequence for degradation. Viral introduction of shRNA plasmids can induce gene-specific silencing temporarily, long-term, and even inducibly depending on the vector and virus.
- Viral CRISPR-Cas9 tools have been engineered to introduce frameshift mutations in genes of interest, turning expression off permanently.
- Viral vectors can also be used to express proteins. Viral expression cassettes typically contain a promoter (inducible or constitutive), your gene of interest, and often a selectable marker. This vector can express your construct in target cells transiently or constitutively depending on the virus used.
All of these expression control mechanisms require a specific modification of the viral vector for your gene of interest – an shRNA sequence specific to your gene’s mRNA or a sgRNA targeting an exon in your gene of interest, for example. Some customization is required, but many viral constructs have already been built and are available on the Addgene catalog – so check there before building one from scratch!
Why deliver these systems virally instead of by another method? Viral delivery is attractive due to the ease of transduction in difficult-to-transfect cell types ranging from primary to embryonic stem cells. Viral integration of a construct is also reliably higher than traditional transfection and random genomic integration of a plasmid. Viral transduction is also generally less toxic to cells than transfections, preserving cell viability.
Library delivery
Screening libraries, large collections of biological independent variables, are becoming increasingly popular tools with many applications. These libraries include sgRNAs targeting the entire genome, overexpression of all human transcription factors, barcoded libraries to trace cell lineage, and so much more! Libraries allow you to test thousands of elements simultaneously in your system instead of just one at a time. Many of these libraries are packaged and delivered with viral vectors instead of a traditional transfection method. This is because viral systems have a high infection frequency and the viral titer (the number of viral particles capable of infecting a host cell) can be tuned so that the copies of library cargo delivered are controllable. For many screening applications, it is essential to deliver a single copy of your library cargo to isolate the effects of each variable being manipulated. Thus, the adjustable nature of viral libraries makes them the ideal vector for the job.
Therapeutic applications
Vaccine development
Attenuated and inactivated viruses pioneered vaccine development well over a hundred years ago. Since then, medical technology has changed a lot, but viruses have stuck around. Vaccine development is moving away from delivery of the actual virus to which immunity is desired. Instead, components of the virus are now delivered in other forms (mRNA, DNA, etc.) to raise immunity. Viral vectors are being explored as the delivery mechanism for increasingly popular nucleic acid-based vaccines. These vectors provide several advantages over traditional vaccines, including 1) cellular responses in addition to antibody response 2) very high immunogenicity 3) and long-lasting immune response even after a single dose.
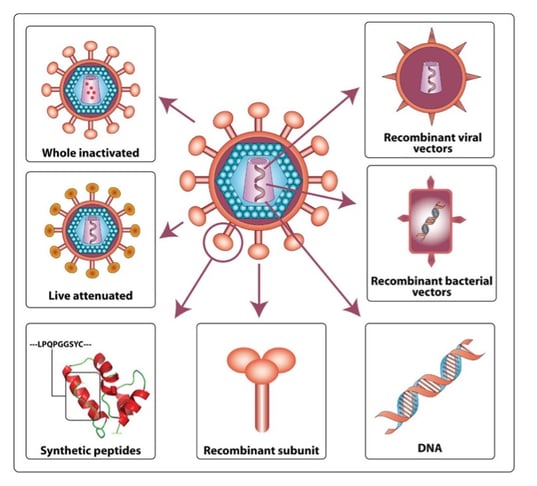
Vaccine development strategies. Image courtesy of Gorry et. al, 2007. |
Clinical applications
Viruses in vaccines helped pave the way for their use in other clinical applications. In the last decade, viral applications in cancer treatment and gene therapy have also been in the limelight. The FDA approved the first oncolytic viral therapy in 2015 to treat melanoma, and many more are in the pipeline. Viruses, specifically AAV, are also being exploited as delivery mechanisms for gene therapy. The tractability of AAV, low immunogenicity, and cell-specific targeting features make them an ideal delivery system for human patients. Multiple viral-based gene therapies have already been approved by the FDA, and this mode of delivery has a lot of clinical promise moving forward.
What viruses can do for you
Whether you are interested in basic science or clinical therapy—viruses could further your efforts. And Addgene is here to help! We offer a variety of viral vectors, viral services and even a Viral Vectors eBook to get you started. Ready to plan your next (or maybe first!) viral experiment?
Click Here to Find Viral Vector Resources at Addgene!
References and Resources
References
Travieso T., et al. The use of viral vectors in vaccine development. Vaccines. 7, 75 (2022). DOI: https://doi.org/10.1038/s41541-022-00503-y
Gorry PR, McPhee DA, Verity E, Dyer WB, Wesselingh SL, Learmont J, Sullivan JS, Roche M, Zaunders JJ, Gabuzda D, Crowe SM, Mills J, Lewin SR, Brew BJ, Cunningham AL, Churchill MJ. Pathogenicity and immunogenicity of attenuated, nef-deleted HIV-1 strains in vivo. Retrovirology. 2007 Sep 23;4:66. doi: 10.1186/1742-4690-4-66. PMID: 17888184; PMCID: PMC2075523.
Bulcha, J.T., et al. Viral vector platforms within the gene therapy landscape. Sig Transduct Target Ther 6, 53 (2021). DOI: https://doi.org/10.1038/s41392-021-00487-6
Additional Resources on the Addgene Blog
- AAV: A Versatile Viral tool for Gene Expression in Mammals
- Listen to Connie Cepko Discuss Gene Therapy with AAV
- Adenoviral Delivery of CRISPR/Cas9 Aims to Expand Genome Editing to Primary Cells
Additional Resources on Addgene.org
Topics: Viral Vectors, Viral Vectors 101





Leave a Comment