Addgene has a growing catalogue of viral preps available to request. In this post, we’ll walk you through how we produce both our AAV preps and our lentiviral preps.
Viruses are generated using standard methods that have been optimized for each specific virus in order to generate high quality preparations. After production, all virus preps are titered and subjected to quality control by Addgene before being distributed to customers. Details about our production protocols, titering methods, and quality control are described below.
AAV
Production
AAV distributed by Addgene has been produced either in-house by Addgene scientists or through collaboration with viral vector manufacturing facilities, such as the University of Pennsylvania Vector Core. Transfections are performed using the transfer plasmid, a plasmid encoding rep and serotype-specific cap genes, and a plasmid encoding adenoviral helper sequences.
AAV preparations are purified by iodixanol gradient ultracentrifugation and concentrated to purity and titers adequate for in vivo studies. Preparations are then aliquoted and stored at -80 °C.
Titer
Titering is either performed by Addgene or by the University of Pennsylvania Vector Core. In general, titering is performed by the facility that produced the viral vector lot. Contact us to determine which facility produced your viral vector lot.
At Addgene, AAV particles are titered by droplet digital PCR (ddPCR) using primers and probes targeting the ITR elements and an internal control of known titer (protocol modified from Lock et al., 2014.) Previous experience suggests that titers obtained using ddPCR are generally two to three fold higher than those achieved using the standard qPCR method.
Titering by the University of Pennsylvania Vector Core is (as of April 2016) also performed by ddPCR.
Quality Control
Addgene ensures high quality viral vectors by optimizing and standardizing production protocols and performing rigorous quality control (QC). The specific QC experiments performed varies for each viral lot. To learn which specific QC experiments were performed on your lot, please contact us.
Full sequencing of the Viral Genome
Next-generation sequencing is performed on viral genomes isolated from the final AAV preparation. Sequencing results are analyzed to confirm the identity and integrity of the viral genome and the absence of unexpected DNA contaminants. The data is also used to determine recombination rates in FLEX/DIO/fDIO constructs.
Endotoxin
Endotoxin contamination in vector preparations can alter the immunogenic properties of the final product, particularly in large animal studies. Endotoxin contamination is minimized by using an endotoxin-free plasmid purification protocol. To minimize the immunogenic properties of the final vector preparation, the quantity of gram-negative bacterial endotoxin is ensured to be less than five endotoxin units per mL. The endotoxin levels are determined using a chromogenic endotoxin detection assay based on the amebocyte lysate method.
Purity
Purity of AAV preparations is assayed by comparing the relative stoichiometric ratios of the viral capsid proteins VP1, VP2, and VP3. Samples of viral preparations are subjected to polyacrylamide gel electrophoresis (PAGE) followed by silver staining or SYPRO Ruby staining and the molecular weight and relative intensity of the viral capsid proteins are analyzed. The abundance of viral capsid proteins as a fraction of total protein present in the sample is also determined and used to determine purity of the AAV preparation.
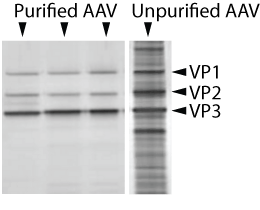 |
| Figure 1: Silver staining of purified and non-purified AAV subjected to gel electrophoresis. Viral capsid proteins VP1, VP2, and VP3 are shown relative to the total protein present in the sample. |
Sterility
Viral vector preparations are added to cells in culture. Three to five days later, cell cultures are inspected for sterility.
Transducibility
Some viral vectors are tested in vitro and in vivo for gene expression and/or function. These data are sometimes reported or posted on the material page for the corresponding catalog item (see maps section for images).
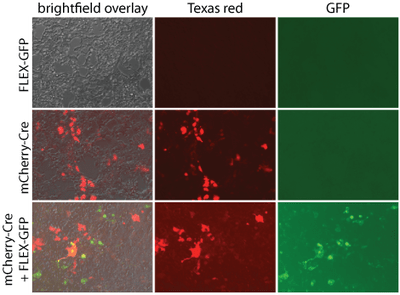
|
| Figure 2: AAV Pro cells were transduced with either pAAV-Ef1a-mCherry-IRES-Cre (55634-AAVrg) alone at 1.7E6 viral genomes (vg)/cell, pAAV-CAG-FLEX-rc [Jaws-KGC-GFP-ER2] (Addgene 84445-AAVrg) alone at 1.1E6 vg/mL, or both. Two days later, Cre-dependent GFP expression was detected with direct fluorescence. GFP was not detected in the absence of Cre. mCherry expression alone was detected. pAAV-Ef1a-mCherry-IRES-Cre was a gift from Karl Deisseroth (Addgene viral prep # 55632-AAVrg). pAAV-CAG- FLEX-rc [Jaws-KGC-GFP-ER2] was a gift from Edward Boyden (Addgene viral prep # 84445-AAVrg). |
Electron Microscopy
The ratio of empty to full (i.e., genome-containing) AAV particles within representative vector preparations was quantified with electron microscopy after negative staining. Empty vector particles can be identified after negative staining and appear darker than full vector particles.
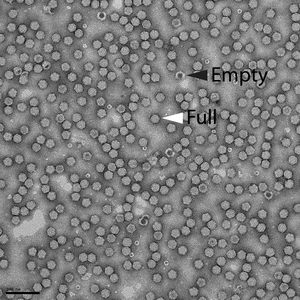
|
| Figure 3: Electron micrograph of AAV vector preparation shows that the vast majority of the vectors consist of full particles (white arrowhead) relative to empty particles (black arrowhead). Scale bar = 100 nm. |
AAV resources
- Browse AAV preps available from Addgene.
- After receiving your virus from Addgene, read our detailed recipient instructions for next steps.
- Plan an experiment using our virus protocols.
- Browse our AAV vector collection.
Lentivirus
Production
Lentiviral preparations ("-LV” catalog items) are produced in 293T cells using the 2nd generation psPAX2 and pMD2.G packaging system developed by the Trono lab. Cell culture medium (OptiPro SFM) containing lentivirus is first cleared by low-speed centrifugation and then by filtration through a 0.45 µm polyethersulfone (PES) membrane. The preparations are then aliquoted, frozen, and stored at -80 °C. Concentrated lentiviral preparations (“-LVC” catalog items) are generated from lentiviral preparations (described above) that are subject to a concentration step prior to being frozen. Specifically, lentiviral particles are collected from the lentiviral preparation by precipitation in 10% polyethylene glycol (PEG) followed by centrifugation. Precipitated pellets containing viral particles are then resuspended in PBS, aliquoted, frozen, and stored at -80 °C.
Titer
All titering is performed on lentiviral preparations that have been stored at -80 °C and thawed. This ensures that any loss of titer associated with the recipient’s initial thaw will be accounted for in our reported titers.
Lentiviral vectors are titered using a lentiviral droplet digital PCR (ddPCR) titration protocol (modified from Wang et al., 2018). Briefly, 293T cells are transduced with serial dilutions of a lentiviral vector, incubated for 72 hours, and genomic DNA is extracted. Primers and probes targeting integrated copies of the lentiviral Rev responsive element (RRE) gene and the cellular ribonuclease P/MRP 30 kDa subunit (RPP30) as a control for normalization purposes are being used.
Quality Control
Mycoplasma
Our 293T cell line was obtained directly from Takara, and is routinely tested for mycoplasma contamination using mycoplasma detection kits. Cell line is maintained for ~20 passages before being discarded and replaced with a new vial of early passage cells. Approximately two weeks post-thaw, cell culture supernatant is tested for mycoplasma contamination. To date, Addgene has never had a case of mycoplasma contamination. In the event of contamination, all of the virus produced in the line will be taken offline and discarded.
Confirmation of the transfer plasmid
Addgene uses a rigorous barcode matching system that follows the sample from the DNA tube all the way to the cleared viral preparation pool.
In addition, the final viral preparation undergoes PCR with primers targeting the transfer plasmid used in the transfection. PCR products are analyzed by gel electrophoresis to ensure the correct banding pattern and sizes.
Sterility
Cells transduced with our lentiviral vectors are routinely checked for signs of microbial contamination.
Purity
When possible, all plasmids used for viral production are propagated in the endA-mutated NEB Stable strain of E. coli. In addition, plasmids are typically prepared using endotoxin-free plasmid purification kits.
.png?width=718&height=503&name=Schematic%20of%20Barcode%20transfer%20system%20(2).png)
|
| Figure 4: Schematic of the barcode matching system and PCR-based confirmation of transfer plasmids used in Addgene’s viral production. Barcoding allows our DNA and virus to be monitored throughout production, ensuring the identity of each sample from the DNA tube all the way to the viral preparation. After production, we confirm the identity of the transfer plasmid in the viral preparation by performing PCR against unique regions of the transfer plasmid. |
Lentivirus resources
- Browse lentiviral preps available from Addgene.
- After receiving your virus from Addgene, read our detailed recipient instructions for next steps.
- Plan an experiment using our virus protocols.
- Browse our plasmid collection of popular lentiviral vectors.
This article was written by multiple Addgenies and most recently updated by Ina Ersing in August 2023.
Resources and references
Resources at the Addgene blog
Find and share AAV Data with Addgene's Data Hub
Digital Droplet for AAV Quantification
Viral Vectors 101: An Introduction to AAV
References
Lock, M., Alvira, M. R., Chen, S.-J., & Wilson, J. M. (2014). Absolute determination of single-stranded and self-complementary adeno-associated viral vector genome titers by droplet digital PCR. Human Gene Therapy Methods, 25(2), Article 2. https://doi.org/10.1089/hgtb.2013.131
Wang, Y., Bergelson, S., & Feschenko, M. (2018). Determination of Lentiviral Infectious Titer by a Novel Droplet Digital PCR Method. Human Gene Therapy Methods, 29(2), 96–103. https://doi.org/10.1089/hgtb.2017.198
Topics: Viral Vectors, Viral Vectors 101, AAV





Leave a Comment