It’s time to choose your own protein purification adventure. You want to purify your favorite protein (YFP). You have two options:
Option #1: Affinity tag purification
You tag YFP and use an affinity column for purification. After binding YFP to the column, you wash several times to remove non-specific proteins, and then elute YFP.
Option #2: Opto-Nanobodies (OptoNBs) purification
You skip adding a tag to YFP and instead use OptoNBs. You fill a column with OptoNB coated beads and wrap the column with blue LED lights. When you switch off the lights, OptoNBs bind YFP and non-specific proteins flow through. To elute YFP, you turn on the blue lights.
Which option do you choose?
Option #2 may sound like fiction, but light-controlled protein purification is now a reality thanks to OptoNBs. Recently designed by the Toettcher Lab, OptoNBs are photoswitchable engineered nanobodies whose binding of their target protein is altered by blue light illumination. This ability to reversibly bind and release a protein of interest in response to light allows for tagless protein purification as well as reversibly regulation of endogenous signaling activity in cells.
Let’s shed some light on the key components of OptoNBs, how they are generated, and their use for protein purification and controlling cell signaling.
Key components of OptoNBs
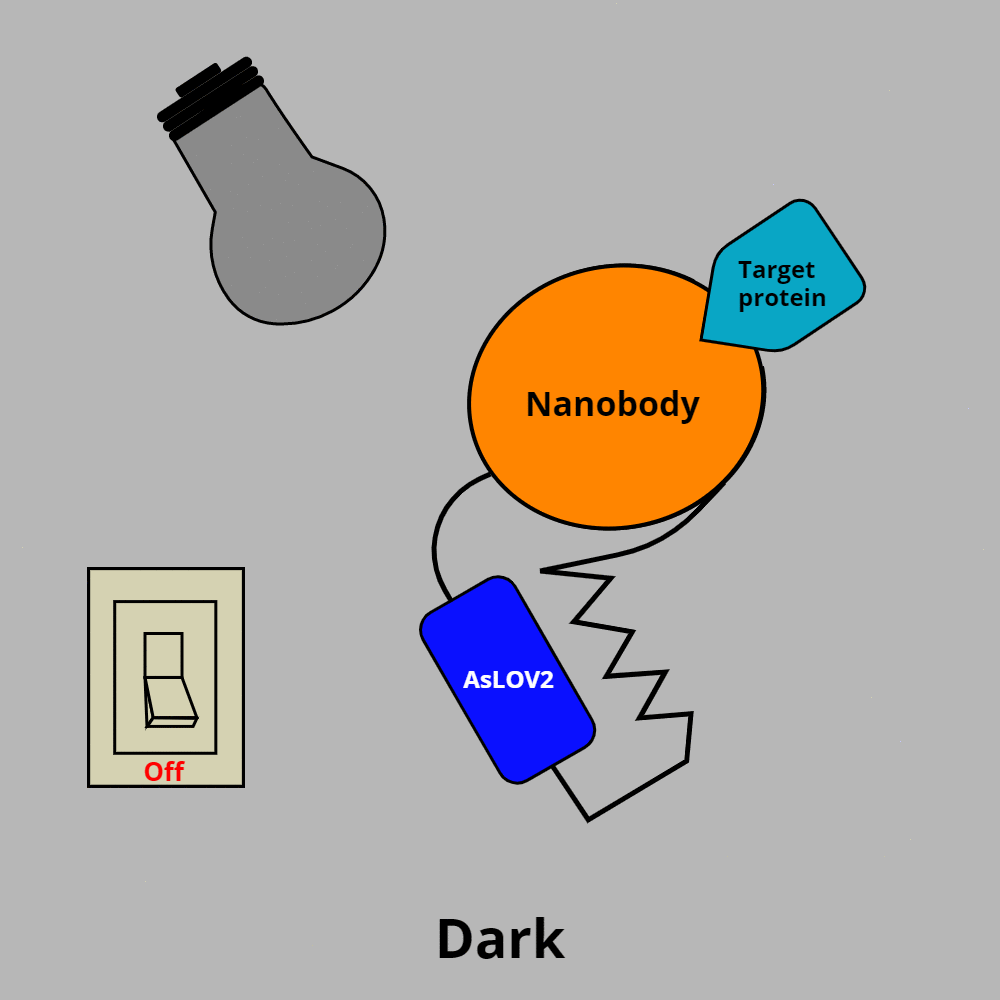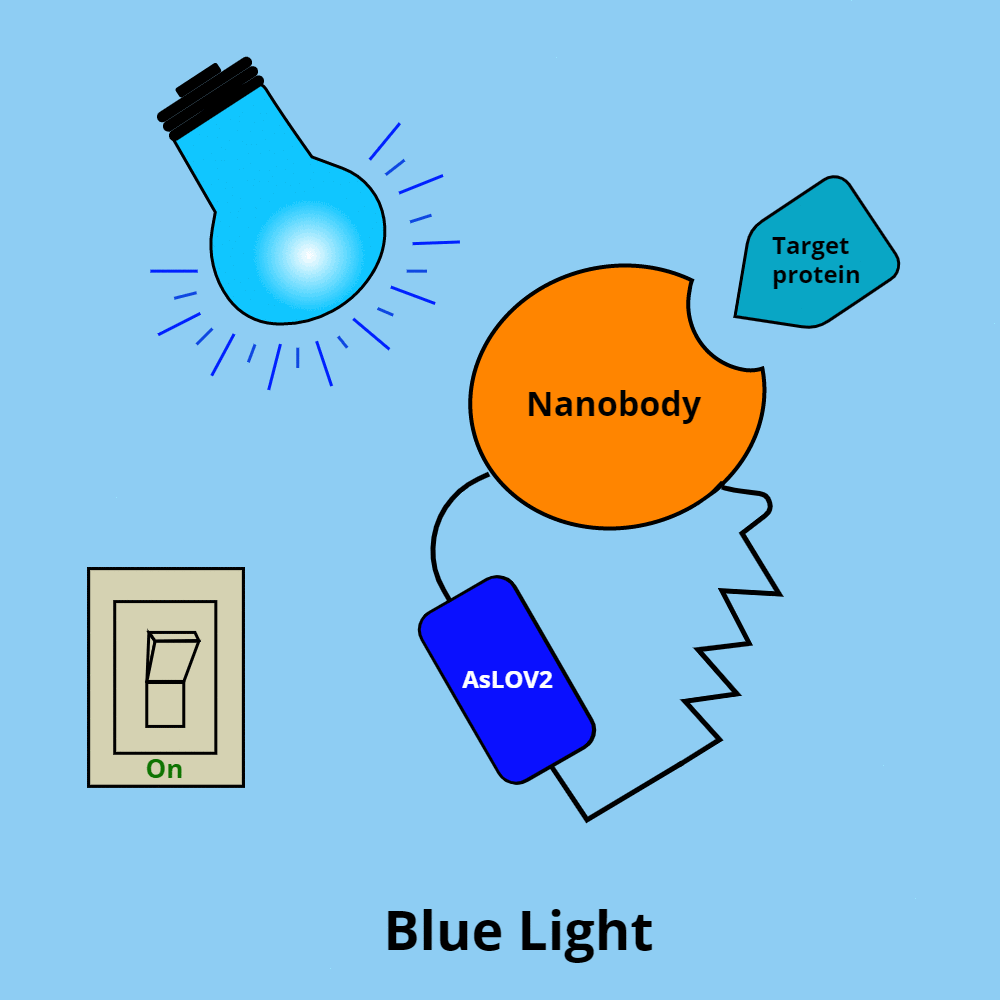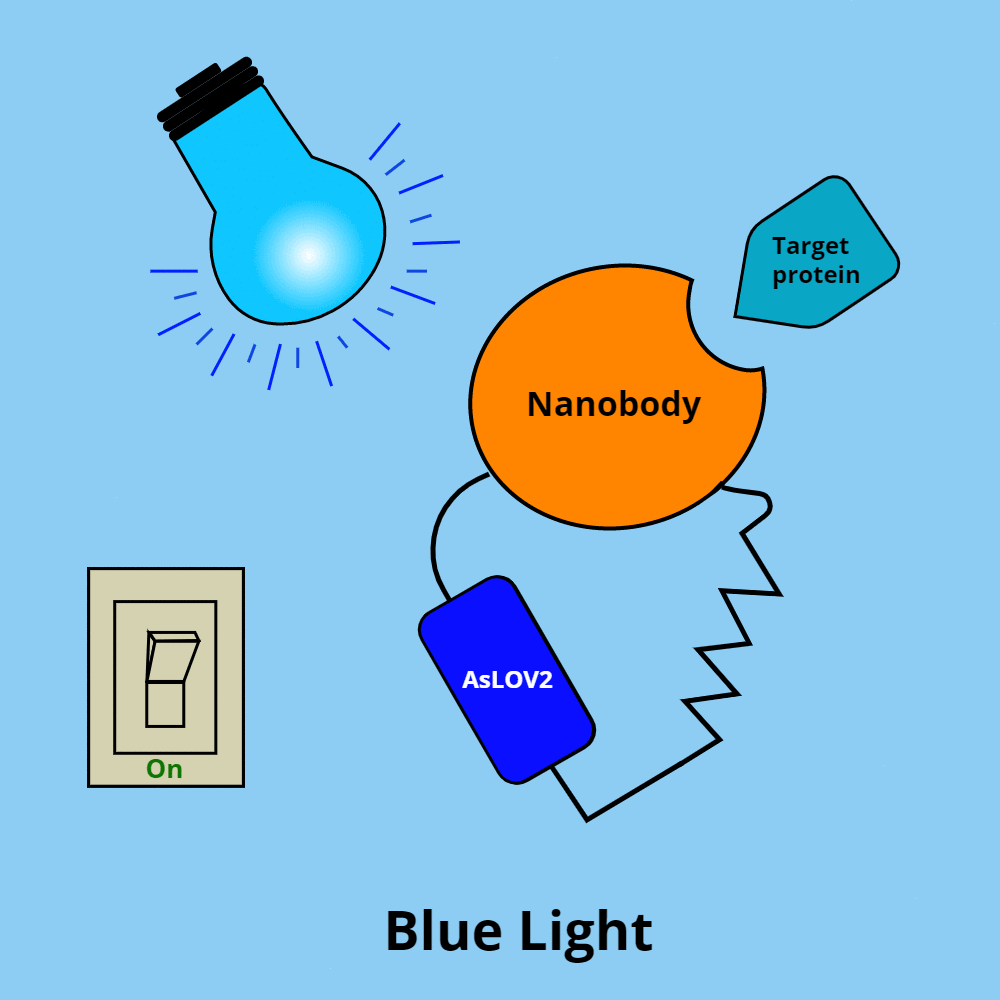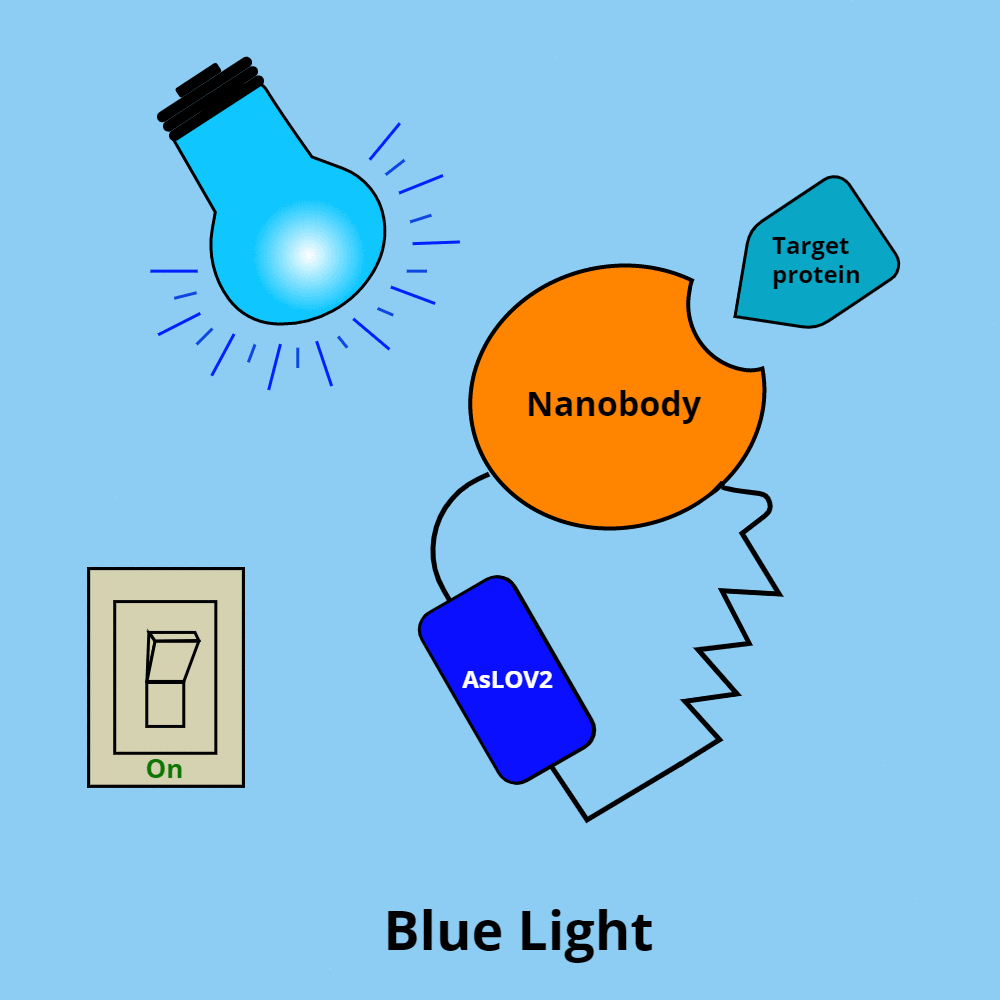
OptoNBs have two key components: an AsLOV2 domain and a nanobody.
- AsLOV2 is a light-oxygen-voltage sensing domain from the oat plant (Avena sativa). In darkness, the AsLOV2 domain folds back on itself in a closed conformation, and in blue light is in an open conformation.
- Nanobodies are a small (~15 kDa), single monomeric variable heavy chain antibody domain.
How do these components interact? When fused together to form an OptoNB, the AsLOV2 domain acts like an on/off switch for the nanobody’s ability to bind its target protein.
Generating OptoNBs
The opening and closing of the AsLOV2 domain allosterically alters the shape and binding ability of its nanobody partner, so it’s important to insert AsLOV2 at a spot in the nanobody that preserves the activity of both AsLOV2 and the nanobody. As a proof-of-principle the Toettcher Lab monitored how AsLOV2 insertion into mCherry or GFP nanobodies affected binding of their target proteins. The surface-exposed loops of nanobodies seemed like a good place to put AsLOV2 because here it was less likely to destroy the nanobody’s function than if inserted into the core of the protein. OptoNBs were also tagged with an infrared fluorescent protein tag which let the team detect its co-localization with a membrane-localized mCherry or GFP protein. With this system, if the OptoNB can bind, it co-localizes with its target at the cell membrane and when the OptoNB can’t bind, it localizes to the cytosol.
To start, the team inserted AsLOV2 into conserved sites in all eight surface-exposed loops of an mCherry nanobody. Only insertion into loops 1 and 6 resulted in light controlled binding, but with opposite effects: insertion into loop 1 caused dark-induced binding while loop 6 insertion caused blue-light induced binding. The lab used this general design scheme to generate two more OptoNBs, one against mCherry and one against GFP. Insertion of AsLOV2 into loop 1 always generated OptoNBs with dark-induced binding, but insertion into loop 6 generated both blue light inducible and dark-inducible OptoNBs. The below table summarizes the results for these experiments.
| AsLOV2 Inertion Site | mCherry #1 | mCherry #2 | GFP |
| Loop 1 | dark-induced | dark-induced | dark-induced |
| Loop 6 | blue-light induced | dark-induced | blue-light induced |
Table 1: Summary of the effect of AsLOV2 insertion site on light-induced binding.
Light controlled protein purification with OptoNBs
The first application for OptoNBs takes us in vitro: light-controlled affinity purification of unmodified proteins. Affinity purification methods typically require a protein to be tagged, which limits these approaches to use with recombinant proteins. Purifying an untagged protein with a flip of a light switch would improve this process.
The team used nickel-coated agarose beads coated with his-tagged OptoNBs. OptoNB coated beads were then imaged while cycling between dark and blue light which revealed a blue light-dependent shift in mCherry fluorescent to the surface of the bead, suggesting that OptoNBs still bound their target protein even when stuck to the surface of a bead.
Using OptoNBs to control cell signaling
The second application for OptoNBs takes us into cells: light-based control of signaling pathways. This is particularly exciting because using light to control signaling provides greater spatiotemporal resolution than pharmacological or genetic manipulation of the signaling pathway.
As a proof-of-principle, the team created an OptoNB to control the Ras/Erk signaling pathway, which is often over-activated in cancer due to its involved in cell proliferation and differentiation.
Since Ras is a membrane localized GTPase, the team fused a mCherry OptoNB to the catalytic domain of the Sons of Sevenless (SOS), a guanine nucleotide exchange factor that activates Ras. They expressed this OptoNB-SOS fusion protein in cells that expressed membrane-localized mCherry. With darkness, SOS binds Ras and initiates the extracellular signaling-regulated kinase (ERK) signaling cascade. This signaling is a lot like a game of telephone, until the message reaches the nucleus where gene expression changes lead to cell proliferation and differentiation. The lab confirmed this OptoNB-SOS activated Ras/Erk signaling by using a fluorescent reporter for Erk kinase translocation. These results demonstrate that OptoNBs can be harnessed to regulate signaling pathways such as the Ras/Erk pathway.
What’s next for OptoNBs?
While OptoNBs illuminate a path to light-controlled protein binding, they probably don’t bind your favorite protein yet. More OptoNBs would allow for more proteins to be taglessly purified. More OptoNBs that bind cell signaling receptors would offer more levers to pull to control signaling pathways. For example, if the OptoNB could bind and block a receptor or a ligand’s activity, then a flip of a light switch could control a signaling pathway. Ultimately, development of more OptoNBs would allow for more uses both inside and outside cells.
References
Gil, Agnieszka A., et al. "Optogenetic control of protein binding using light-switchable nanobodies." BioRxiv (2019): 739201.
Additional resources on the Addgene blog
- Learn about using nanobodies to control protein activity
- Read about RANbodies, reporter nanobody fusions
- Learn more about using the secondary nanobody toolbox for immunodetection
Resources on Addgene.org
- Find nanobody expression plasmids here
- Learn more about optogenetics in Addgene's optogenetics guide
Topics: Optogenetics, Antibodies






Leave a Comment