Every few months we highlight a subset of the new plasmids, antibodies, and viral preps in the repository through our hot plasmids articles. Think we missed something smokin'? You can pitch a Hot Plasmids section (antibodies and viral preps welcome!) here.

Here's what you'll find in this post:
CRISPR-Csm: A novel RNA targeting system
by: Susanna Stroik
The Doudna lab’s recent work characterized a new tool for RNA targeting, with a leg up on the alternatives - RNAi and Cas13. CRISPR-Csm doesn’t degrade proximal RNAs nor does it have high levels of off-target effects, both of happen with Cas13. CRISPR-Csm degrades both nuclear and cytoplasmic mRNA with 90-99% knockdown efficiency across the 11 candidate transcript targets analyzed.
CRISPR-Csm presents an attractive alternative for nuclear-RNA targeting, where siRNAs are ineffective and Cas13 has steep off-target costs. Additionally, this system also demonstrated the ability to be used as an endogenous RNA imaging tool.
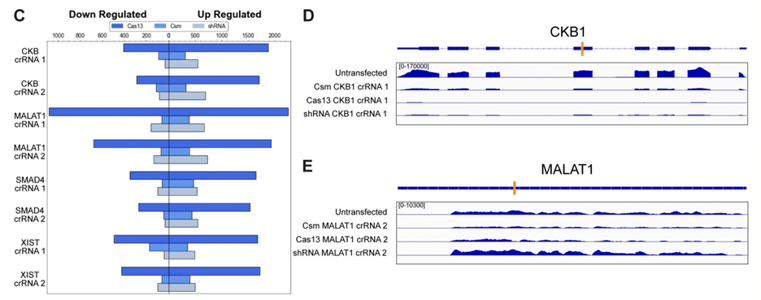
|
| Fig. 1: Csm, shRNA, and Cas13 were compared head-to-head for gene expression changes of non-target genes and RNA-seq of a nuclear (MALAT1) and cytoplasmic (CKB1) RNA target. Adapted from Figure 3 Colognori et al 2022. |
Find CRISPR Csm plasmids here!
Colognori D, Trinidad M, Doudna JA. Precise transcript targeting by CRISPR-Csm complexes. Nat Biotechnol. 2023 Jan 23. doi: 10.1038/s41587-022-01649-9. Epub ahead of print. PMID: 36690762.
Heat-inducible lentiviral CAR constructs
by: Brian O'Neill
A clever system for reducing the on-target off-tumor activity of CAR–T cells was recently devised by the Yingxiao Wang lab. The crux of the system is a set of heat-inducible lentiviral CAR constructs, the most basic of which is depicted here:
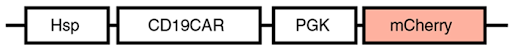
The heat-inducible Hsp (heatshock promoter) drives expression of the tumor-seeking CAR (chimeric antigen receptor), whereas a ubiquitous promoter drives expression of an mCherry reporter. After using mCherry for FACS sorting of the weaponized T-cells (CAR-T cells) - and then delivering them to a tumor - they used focused ultrasound (FUS) to heat the tumor area, as the image below shows:
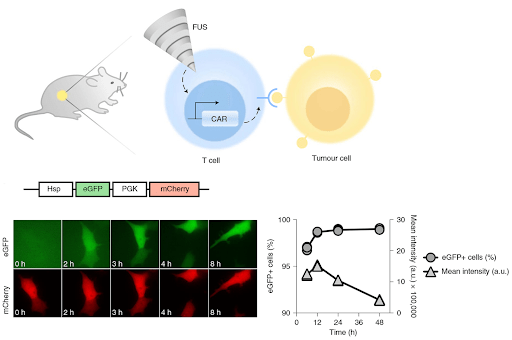
This system ablates tumors just as effectively as standard CAR-T cells, but it reduces the off-target effects caused by T cell migration. CAR expression (or the eGFP proxy for CAR) is elevated at stable levels for 6 days - after just 15 minutes of FUS. Similar constructs to the one above can be made for expressing your favorite T-cell receptor, or perhaps any other tool that can benefit from region-specific, inducible expression.
Find heat-inducible lentiviral plasmids here!
Wu Y, et. al. Control of the activity of CAR-T cells within tumours via focused ultrasound. Nat Biomed Eng. 2021 Nov;5(11):1336-1347. doi: 10.1038/s41551-021-00779-w. Epub 2021 Aug 12. PMID: 34385696; PMCID: PMC9015817.
Meet anti-GFP [N86/8R]!
by: Ashley Waldron
Anti-GFP [N86/38.1R] was among the first batch of antibodies we produced at Addgene and, as you might suspect, it has been one of our most commonly requested antibodies. But sometimes, one antibody per target just isn’t enough and we recently released Anti-GFP [N86/8R] as another option for targeting GFP. But why might you need a second GFP antibody?
Similarities:
- Generated against full length GFP and converted to recombinant form by the NeuroMab Facility
- Isotype IgG2a
Differences:
- Distinct variable domains that recognize different epitopes on GFP
Monoclonal antibodies can yield relatively low signal in immunoassays due to only having one binding site per protein. Having multiple antibodies allows you to use them in parallel to help amplify your signal - giving you the benefit of a polyclonal, with the reproducibility of a (recombinant) monoclonal. Another reason is that some antibodies may just not perform as well in one application as another, so it is always best to have options.
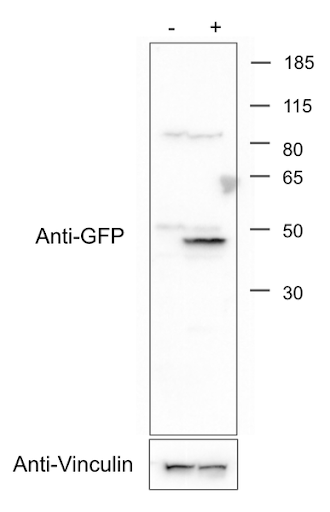
|
| Fig. 2: Addgene’s western blot for mEGFP-HRas (from Plasmid 18662). See the Applications section on the Anti-GFP [N86/8R] page for more details. Image credit: Addgene. |
Andrews NP, et. al. A toolbox of IgG subclass-switched recombinant monoclonal antibodies for enhanced multiplex immunolabeling of brain. Elife. 2019 Jan 22;8:e43322. doi: 10.7554/eLife.43322. PMID: 30667360; PMCID: PMC6377228.
Hyperfolder YFP- a chemically stable tool for microscopy
by: Susanna Stroik
Microscopy techniques have been moving at the speed of light over the last decade, but the fluorescent proteins (FPs) necessary for many of these applications have lagged. Fixation, chemical staining, and light exposure can negatively affect FPs during these processes. The Liu and Petsko labs recently engineered hyperfolder YFP (hfYFP), a FP well-suited for the demands of advanced microscopy applications such as super resolution, electron, and expansion microscopy. hfYFP is not only brilliantly bright, but it also withstands denaturing conditions, high temperatures, and other chaotropic conditions better than most FPs. The group also tested hfYFP in expansion microscopy as well as correlative light and electron microscopy and observed high fluorescence resilience to these preparations. After crystalizing the hfYFP structure, the group rationally designed GFP variants with similar capabilities. If you’re looking for a super stable FP for your next microscopy experiment, consider hfYFP and LSSmGFP.
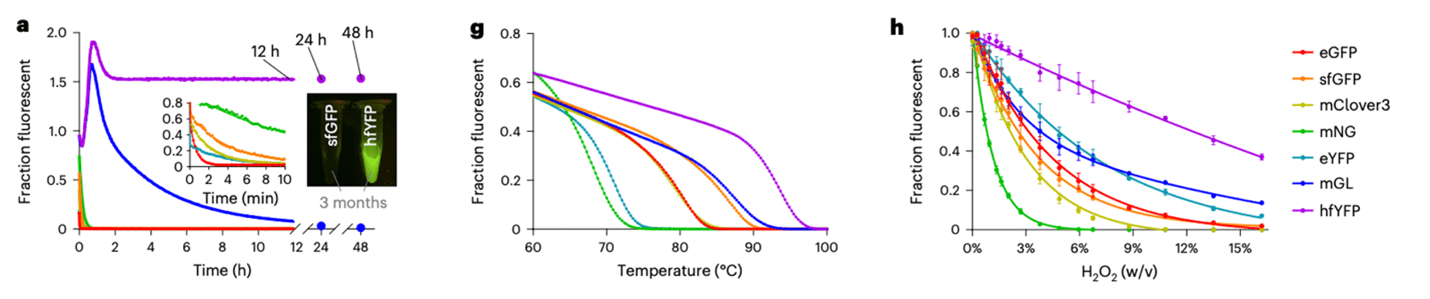
|
| Fig. 4: hfYFP retains folding and fluorescence in high molarity GdnHCl, at high temperatures, and in the presence of oxidizing agents. Adapted from Fig. 2 Campbell et al, Nature Methods |
Campbell, B.C., et al. Chemically stable fluorescent proteins for advanced microscopy. Nat Methods 19, 1612–1621 (2022). https://doi.org/10.1038/s41592-022-01660-7
Topics: Hot Plasmids






Leave a Comment