A few months ago, we shared an introduction to immunofluorescence (IF) - a common method for visualizing molecules of interest within a cell or tissue. In that introduction, we broke down the method into six general steps and outlined the considerations to be made during each step. Now that you know the basic principles of IF, we’ll take a slightly deeper dive into those steps and provide you with more details to help you plan and optimize your own protocols. Here we’ll start with just the first couple of steps - mainly, fixing and permeabilizing.
Fixation
What is the point?
Fixation allows you to freeze your samples in a given state or point in time. You want to halt degradative processes while maintaining the structure of your samples, the relationships between cellular components, and the accessibility of your target epitope(s). To achieve these goals, researchers commonly use one of two types of fixatives - chemical cross-linkers and organic solvents.
Chemical Cross-Linkers
Chemical cross-linkers work by (as the name suggests) cross-linking cellular proteins. Cross-linking is a chemical reaction that covalently joins two molecules and it is great for preserving your samples and maintaining cellular and tissue morphology. Formaldehyde is the most commonly used cross-linker for IF. However, you might come across a couple other terms that can cause some confusion.
- Paraformaldehyde: Many IF protocols call for paraformaldehyde (PFA) as the fixative. PFA is a polymer of formaldehyde that, on its own, is not a fixative. However, when PFA is dissolved in solution and heated, it depolymerizes, producing formaldehyde. Often people will call the resulting solution PFA, but it’s really just formaldehyde.
- Formalin: You may also come across formalin, which refers to a saturated solution of formaldehyde (saturated being 37%). Formalin solutions often contain methanol as well, in order to slow the polymerization of formaldehyde.
Note: Formaldehyde in solution will naturally polymerize and lose its ability to cross-link proteins. As such, you will get the best results if you use fresh formaldehyde solutions.
Along with preserving your sample, cross-linking can block the epitope of some targets, preventing your primary antibody from binding (Fig.1). The level of cross-linking that occurs depends on the incubation time and temperature, so you may be able to recover good antibody binding by tweaking those parameters, but in some cases your antibody may just not be suitable for use with this type of fixation*.
Organic Solvents
Organic solvents, generally acetone, methanol, or ethanol, work by dehydrating and precipitating cellular components. This method of fixation is good for epitopes that are sensitive to cross-linking, but it doesn’t preserve sample structure quite as well as a cross-linker. This is because organic solvents can be quite harsh - lipids and soluble proteins can be lost during fixation with these chemicals, which impacts sample structure and could also cause your target to be washed away. Furthermore, these chemicals can also alter the structure of proteins, which could disrupt your target epitope (Fig. 1). So, while organic solvents may improve antibody binding for some targets, they can destroy it for others. That said, organic solvents’ impact on lipids means that they are able to permeabilize your sample while fixing it, which may be a good thing depending on your experiment.
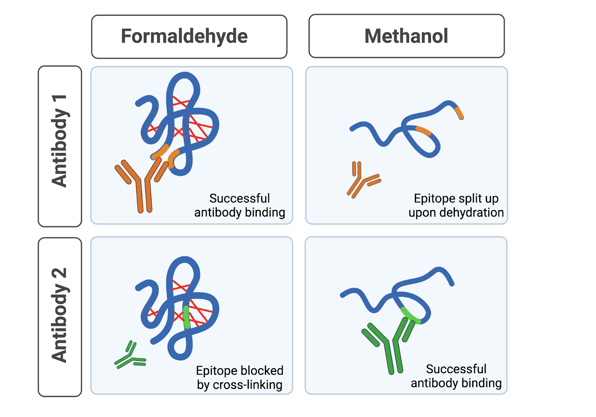 |
| Figure 1: Choice of fixation method impacts antibody binding. Formaldehyde cross-links the protein (red lines), which maintains structural epitopes (orange region), but it can block epitopes hidden within the folded protein (green region). Methanol dehydrates the protein, which can disrupt protein structure and thus structural epitopes, but can reveal linear epitopes previously hidden within the protein. Created with BioRender. |
Permeabilization
What is the point?
Antibodies are large proteins that need a little help crossing cell membranes as they are not able to diffuse across natively. Permeabilization essentially punches holes in the cell membranes of your sample to allow antibodies better access to intracellular targets. If your target is already on the extracellular side of the membrane, then this step may not be necessary. In fact, you may get cleaner results by not permeabilizing as you’ll prevent binding to any target protein that is still on the inside of the cell. However, assuming you do need to permeabilize, you have a few options for what reagents to use - organic solvents and detergents.
Organic Solvents
As mentioned above, organic solvents can be used to simultaneously fix and permeabilize a sample. But you can also use organic solvents after fixing your sample with a cross-linker. This strategy gives you the benefits of both reagents - cross-linkers better preserve sample morphology, while organic solvents can reveal otherwise hidden epitopes. However, keep in mind that you will still contend with many of the same drawbacks to each reagent type, so this approach is not suitable for all antibodies.
Detergents
Another common family of permeabilization reagents are detergents, such as Triton X-100, Tween-20, or saponin. Each detergent has different chemical properties that will impact the level of permeabilization and membrane integrity (Fig. 2). For example, saponin interacts with cholesterol in cell membranes and leaves many membrane-associated proteins in place. In contrast, Triton X-100 and Tween-20 are examples of non-ionic detergents that interact with lipids and proteins in membranes and permeabilize non-selectively. This non-selectivity is helpful when trying to pass tough-to-permeabilize membranes and these detergents avoid some of the negative effects caused by organic solvents. However, detergents can lyse cells or cause you to lose soluble proteins from the sample, especially if left on for too long or used at too high a concentration.
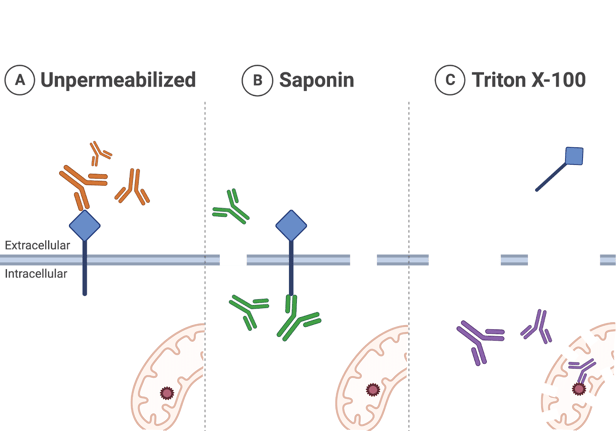
|
| Figure 2: Examples of different permeabilization strategies and when to use them. A) Not permeabilizing is suitable for antibodies that bind to the extracellular portion of a protein. B) Permeabilizing with the selective detergent, saponin, keeps membrane-associated proteins in place, while allowing antibodies into the cell where they could bind to intracellular epitopes. C) Permeabilizing with a non-ionic detergent, like Triton X-100, can lose membrane-associated proteins, but it is better at permeabilizing organellar membranes. Created with BioRender. |
How do I choose?!
Ok, so now you know some of the possible types of fixatives or permeabilizers, but how do you decide which ones to use? Here are some tips for making those decisions:
- Always always always look at your antibody datasheet to see if it has been tested in IF and if a protocol is available. When possible, try to start with conditions and reagents that have already been used successfully.
- If there isn’t information on how to use your specific antibody for IF, look for general IF protocols for your sample type. Starting with a protocol that usually works in your system and then optimizing from there is a good option.
- Consider your target - if you are studying a membrane protein and you want to minimize the risk of it being lost from your sample, start by fixing with formaldehyde and permeabilizing with saponin. However, if you are not worried about washing away your target (for example, some cytoskeletal components) and you want to fix and permeabilize in one step, then methanol treatment could be an effective strategy. (This article on fixation has some more examples to help choose which fixative to use!)
Ultimately, the only way to know if a method will work is to try it. As you embark on your next IF adventure, just remember that even though these early steps don’t even involve antibodies, they are still critical to the outcome of your IF experiment and deserve careful thought and attention.
* There are other methods for recovering epitope availability following cross-linking fixation; they require an additional step generally referred to as Antigen Retrieval. I chose not to get into antigen retrieval here because it’s not quite as ubiquitously required for IF as fixing and permeabilizing.
References and resources
References
Brown-Harding H (2020) Fixation artifacts and how to minimize them. FocalPlane. https://focalplane.biologists.com/2020/07/07/fixation-artifacts-and-how-to-minimize-them/
Goldenthal KL, Hedman K, Chen JW, et al (1985) Postfixation detergent treatment for immunofluorescence suppresses localization of some integral membrane proteins. Journal of Histochemistry & Cytochemistry 33:813–820. https://doi.org/10.1177/33.8.3894499
Im K, Mareninov S, Diaz MFP, Yong WH (2018) An Introduction to Performing Immunofluorescence Staining. Methods in Molecular Biology. Springer New York, pp 299–311. https://doi.org/10.1007/978-1-4939-8935-5_26
Rolls G (2022) Process of Tissue Fixation & the Nature of Fixatives in Histology. Leica Biosystems. https://www.leicabiosystems.com/us/knowledge-pathway/fixation-and-fixatives-1-the-process-of-fixation-and-the-nature-of-fixatives/
Additional resources on the Addgene blog:
- Antibodies 101: Introduction to Immunofluorescence
- Antibodies 101: Epitope Availability
- Antibodies 101: Introduction to Antibodies
Resources on Addgene.org
- Find recombinant antibodies at Addgene
- Find plasmids encoding recombinant antibodies at Addgene






Leave a Comment