Chemogentic and optogenetic technologies have pushed the boundaries in neuroscience by granting targeted control over neuronal activity. While they serve similar purposes, both techniques offer researchers different advantages and limitations.
The four main factors in which chemogenetics and optogenetics differ are:
- Timing
- Targeted manipulation
- Controlling stimulation
- Invasiveness
Choosing the best set of tools therefore depends on what you are looking to study.
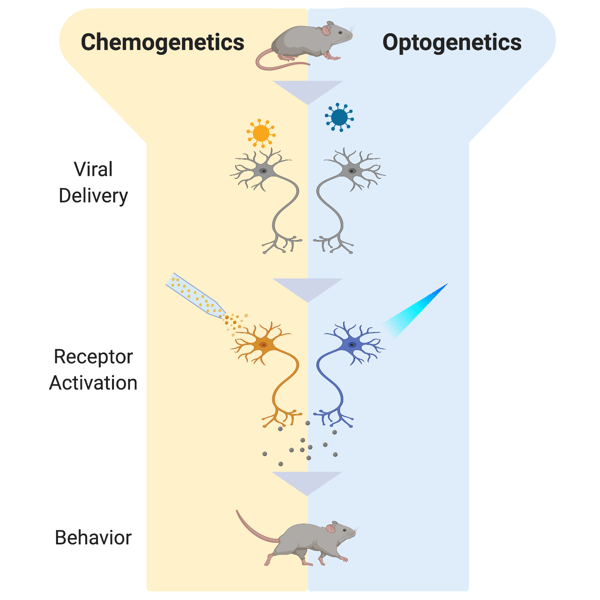 |
| Figure 1: A schematic showing the differences in chemogenetics and optogenetics in a mouse experiment. Viral delivery is the same in both cases, but activation depends on small molecule activation (chemogenetics) or light (optogenetics). |
Timing
Optogenetics offers a high degree of temporal resolution as receptors can be turned on and off simply with the presence or absence of the correct wavelength of light. This response is immediate, on the scale of milliseconds, and can precisely link a neuron's activation to behaviors. Scientists continue to engineer opsins to operate at increasingly faster kinetics. The opsin ChETA, for example, is a ChR2 opsin mutated to have faster off kinetics and allow neurons to repolarize faster.
Chemogenetics does not offer the same level of control over timing. Because chemogenetics relies on chemical activation and the diffusion of drugs throughout the body, the onset of receptor activation takes minutes. As a result, it is not possible to elicit an immediate response or track the exact time of activation. Receptor activity is also drawn out as drugs can remain present until cleared by the body. Administering the drug once can result in several hours of neuronal excitation or inhibition. This sustained activation period is beneficial for use in therapeutic or behavioral applications where drawn out effects are required.
Targeted manipulation
Both optogenetic and chemogenetic tools can have their expression targeted to specific tissues, cell types or even subcellular regions of a neuron. Optogenetic approaches excel at discrete cell or tissue-subregion targeting down to the limits of light delivery. Chemogenetic approaches excel at broad tissue or system-wide targeting, as the ligand can reach target cells throughout the body.
Controlling Stimulation
Some studies rely on delivering reversible or varied amounts of stimulation to receptors. Optogenetics offers better control over these factors as the light source can be easily and quickly manipulated or turned off. In chemogenetics, while different concentrations of ligands can be injected, this is not as precise and the offset of stimulation is gradual.
Invasiveness
Both optogenetic and chemogenetic tools need to be delivered to cells, commonly through viral delivery by AAV. You can find AAV tools for optogenetics and chemogenetics on the Addgene website.
Optogenetics also requires that light of the correct wavelength reach receptors in the brain. One way to address this is by working with organisms with light accessible brains, such as zebrafish larvae. Mouse studies, however, require a permanent intracranial implant to be surgically installed to allow for light delivery.
Chemogenetics poses the advantage of being less invasive and more flexible as it relies on designer ligands that can be simply introduced by injection. The main challenge in using ligands is ensuring the agonist is able to cross the blood brain barrier while also being selective and effective. A new ligand from the Bryan Roth lab and Takafumi Minamimoto’s lab known as deschloroclozapine (DCZ) has been developed to meet these requirements.
TL;DR
If precise spatiotemporal control of a small subset of neurons is necessary, use optogenetics. If broad control of whole circuits or tissue-wide systems is necessary, use chemogenetics. Note that, as with all tools, both approaches suffer from unique sets of off-target side-effects that should be carefully controlled for in experimental design.
| Optogenetics | Chemogenetics | |
| Timing | Fast activation/deactivation | Prolonged activation |
| Targeting | Further restricted to illuminated region | Impacts all cells expressing receptors |
| Invasiveness | Mice require permanent intracranial implant | Noninvasive |
| Controlling Stimulation |
Exogenous |
Endogenous |
| Unique advantages | Mapping the brain Dissecting circuits |
Studying GPCR pathways |
Acknowledgements
We thank the Bryan Roth lab for feedback on this article and sharing their expertise with us.
References
Urban DJ, Roth BL (2015) DREADDs (Designer Receptors Exclusively Activated by Designer Drugs): Chemogenetic Tools with Therapeutic Utility. Annual Review of Pharmacology and Toxicology 55:399–417 . https://doi.org/10.1146/annurev-pharmtox-010814-124803
Vlasov, K., Van Dort, C. J., & Solt, K. (2018). Optogenetics and Chemogenetics. In Methods in Enzymology (pp. 181–196). https://doi.org/10.1016/bs.mie.2018.01.022
Additional resources on the Addgene blog
- Read more about optogenetics
- New to viral vectors? Browse our Viral Vectors 101 blog posts
- Learn more about precise targeted manipulation
Resources on Addgene.org
- Browse all viral vectors available at Addgene
- Visit our viral vectors guide
- Find our chemogenetics and optogenetics science guides
Topics: Optogenetics, Viral Vectors, Chemogenetics








Leave a Comment