Optogenetics is a neuroscience method that lets you fire neurons with the flick of a light switch. Neurons are not typically persuaded to fire when light is shined on them, but the expression of light-gated ion channels such as channelrhodopsins (ChRs) makes them light-responsive. When light shines on excitatory channelrhodopsin-expressing neurons, the channelrhodopsins respond by opening and allowing an influx of ions into the neuron which generates an action potential (Figure 1). This light-induced excitation can take place in a tissue culture dish but can also happen in real-time inside a mouse’s brain.
But the brain is a tricky organ to access, which often forces scientists to use invasive measures to perform in vivo optogenetic experiments. Intracranial injections of AAV are commonly used to deliver channelrhodopsins to the brain. This delivery route concentrates lots of channelrhodopsins in one region of the brain, which is needed since current channelrhodopsins tools have low conductance, but also confines channelrhodopsin activation to a small volume of brain tissue (~1 mm3). Current channelrhodopsins also need high-intensity light for activation which requires implantation of fiber-optic cables into the brain.
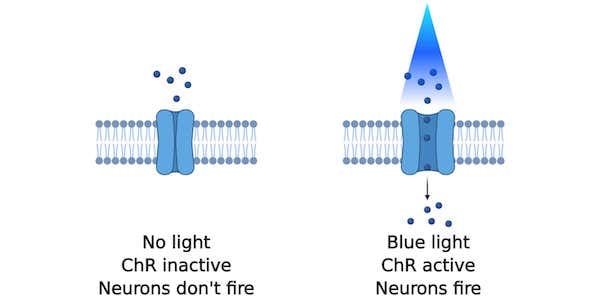 |
| Figure 1: With no blue light, the channelrhodopsin remains inactive and neurons don't fire. With blue light, ions flow into the neuron and causes it to fire. Created with BioRender.com. |
To make channelrhodopsin-based optogenetics minimally-invasive, Frances Arnold’s lab and Viviana Gradinaru’s lab used machine learning to design new channelrhodopsins that are more light sensitive and have enhanced conductance compared to current channelrhodopsins. Their new channelrhodopsins, called ChRgers, make the brain a little easier to access with optogenetics.
Challenges of engineering channelrhodopsins
There are three main challenges to engineering channelrhodopsins to have improved photocurrent properties:
- The sequence and structures that determine membrane expression and plasma membrane localization of transmembrane proteins, like rhodopsins, are highly constrained and poorly understood.
- It’s hard to design new channelrhodopsins with high-throughput proteins engineering methods, like directed evolution, because channelrhodopsins are typically evaluated with low-throughput methods such as patch-clamp electrophysiology, a technique that’s used to measure electric current of ion channels in living cells.
- Multiple properties of a channelrhodopsin, such as cell localization, kinetics, and spectral properties, must be optimized simultaneously in order to generate a channelrhodopsin that’s successful in vivo.
Using machine-learning to overcome channelrhodopsin design challenges
To overcome these challenges, the Arnold and Gradinaru Labs developed a machine-learning model to help them construct “designer” channelrhodopsins with desired characteristics. The model was trained to predict if a channelrhodopsin sequence would result in a functional channelrhodopsin, as well as predict the channelrhodopsin’s photocurrent strength, wavelength sensitivity, and off-kinetics.
This model was then used to predict which channelrhodopsins from a library of ~120,000 predicted channelrhodopsin sequences would have photocurrent properties best suited for less-invasive optogenetics experiments. The sequences in this library were designed using structural information about parental channelrhodopsins to create recombinant channelrhodopsin sequences. The model first removed sequences from the library predicted to be non-functional, reducing the library to 1,161 sequences. Thirty of these remaining sequences were selected for characterization with patch clamp electrophysiology. All thirty were functional and their measured photocurrent amplitude, wavelength sensitivity, and off-kinetics correlated with the model’s predictions.
Three top performers, named ChRger1, ChRger2, and ChRger3, were then characterized further by expression in cultured neurons and mouse brain slices collected following intracranial injection of an AAV expressing a ChRger. In both experiments, neurons expressing ChRgers produced stronger photocurrents at low light intensity than existing channelrhodopsins CoChR and/or ChR2(H134R).
Designer channelrhodopsins paired with a systemic AAV allow for minimally-invasive optogenetics
While ChRgers performed well in a tissue culture dish, that doesn’t guarantee their success in vivo. Therefore, the Arnold and Gradinaru Labs expressed ChRgers in mice to see if they were suited for in vivo minimally invasive optogenetics. Both experiments delivered ChRgers by intravenous injection of PHP.eB, an AAV capsid capable of broad transduction of the nervous system which eliminates the need for intracranial injections. When packaged in PHP.eB, ChRgers and ChR2(H134R) both reach the brain, but individual neurons do not express as many copies of the ChRs as compared to direct injection. This lower multiplicity of infection (MOI) makes it challenging for ChR2(H134R) to function because it’s lower conducence requires higher expression levels in order to activate a neuron. ChRgers, however, have a higher conductance, so they can still depolarize neurons at lower MOIs.
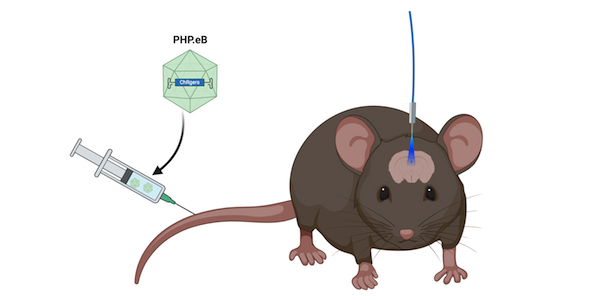 |
| Figure 2: ChRgers are delivered by intravenous injection into mice. A fiber-optic cable allows blue light to reach the brain. Created with BioRender.com. |
In the first experiment, ChRger2 was non-invasively delivered to the dopamine producing neurons in the ventral tegmental area (VTA) of the brain. Since the VTA is located in the midbrain, fiber-optic cannulas were still stereotaxically implanted into the mice’s brains so that light could reach the VTA. Mice were then allowed to “self-stimulate” their VTA dopaminergic neurons by triggering a burst of light that could activate ChRger2 or ChR2(H134R) when they poked their nose into a hole in their cage. Since dopamine is an important neurotransmitter that promotes reward-motivated behavior, if the channelrhodopsins activate the dopaminergic neurons in the VTA, this should propel the mice to increase their nose-poking behavior. Strong nose-poking behavior is exactly what was seen with ChRger2 expressing mice, while ChR2(H134R) expressing mice did not have this self-stimulation response. This result showed that ChRger2 stronger photocurrent properties make it better suited for systemic delivery with PHP.eB compared to the existing ChR2(H134R).
In the second experiment, ChRger2 was delivered and activated with non-invasive methods to the neurons in the right secondary motor cortex (M2). Since M2 is located on the surface of the brain, to deliver light to the channelrhodopsin-expressing neurons, the lab could thin the mice’s skull near the M2 and secure a fiber optic cannula to the skull, leaving the brain intact. When the fiber optic light was turned on, mice that received ChRger2 turned towards the left which is what’s expected if neurons in the right M2 are activated, but mice that received ChR2(H134R) did not turn. Left-turning behavior in ChRger2-treated mice ceased when the light was turned off.
Together these two experiments determined that ChRger2’s improved light sensitivity and higher-photocurrent properties when paired with PHP.eB’s systemic delivery enables minimally-invasive gene delivery and optogenetic excitation. Excitation of neurons deeper in the brain may still require fiber-optic cannula implantation, but altering ChRgers so they can be activated with tissue-penetrating near-infrared light would allow for non-invasive deep brain optogenetics.
TL;DR
The new channelrhodopsins, called ChRgers, make it easier and less invasive to use optogenetics in the brain.
References
Bedbrook CN, Yang KK, Robinson JE, Mackey ED, Gradinaru V, Arnold FH (2019) Machine learning-guided channelrhodopsin engineering enables minimally invasive optogenetics. Nat Methods 16:1176–1184 . https://doi.org/10.1038/s41592-019-0583-8
Additional resources on the Addgene blog
- Read more about the PHP.eB AAV capsid
- Read more about optogenetics
- Learn about the differences between chemogenetics and optogenetics
Resources on Addgene.org
- Browse all viral vectors available at Addgene
- Visit our viral vectors guide
- Find our chemogenetics and optogenetics science guides
Topics: Optogenetics, Viral Vectors, Neuroscience






Leave a Comment