If you’re just getting started using antibodies in your experiments, you may be curious about all of the different kinds of antibodies that are available. One common type of antibody is a monoclonal antibody. But what does that mean, and how do monoclonal antibodies differ from other types of antibodies?
How monoclonal antibodies are made
“Monoclonal” refers to antibodies that are all of the same isotype and specificity. In other words, monoclonal antibodies are derived from a single B cell clone. One common way of producing monoclonal antibodies is from a hybridoma, a fusion between an antibody-producing B cell and a myeloma cell (National Research Council (US) Committee on Methods of Producing Monoclonal Antibodies, 1999). This creates an immortal cell population that will continue to produce the specific antibody that the original B cell produced. Because of this, all of the antibodies recognize a single epitope on the target molecule. Although it’s possible to generate antibodies against many types of molecules including proteins, lipids, and carbohydrates, in this blog post we’ll focus on antibodies that recognize proteins.
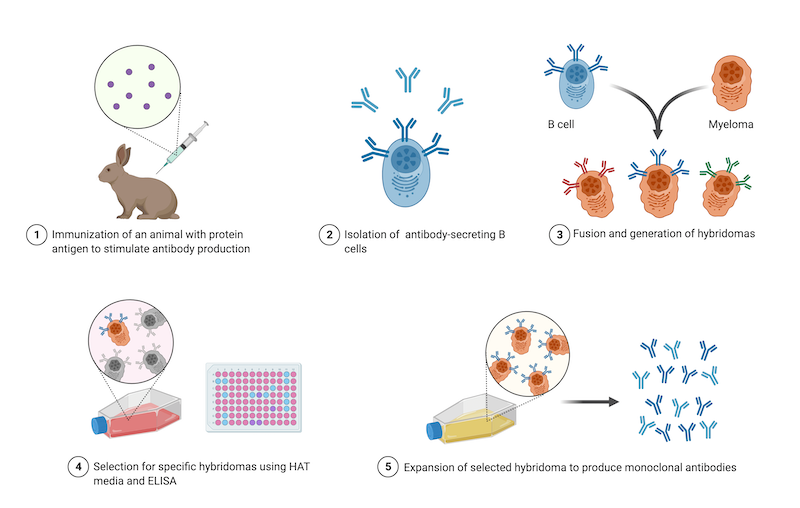 |
| Figure 1: Steps in the generation of hybridomas for monoclonal antibody production. Image created with BioRender.com. |
The antibody-producing B cells that are used to make hybridomas come from animals that were immunized against the target protein. Animals are first injected with a small peptide that represents a short, unique amino acid sequence found in the protein, or, are injected with the whole protein. After isolating the B cells from an immunized animal, those B cells are fused to myeloma cells at a 1:1 ratio and then cultured in a special medium called HAT. This medium selects against unfused myeloma cells and antibody-producing B cells that cannot replicate, meaning that only hybridomas will survive this step. Then, a limiting dilution is performed to isolate individual hybridomas and ELISA is used to identify the hybridomas producing the most specific antibodies. Finally, the selected hybridoma is expanded as a clonal population (Holzlöhner et al., 2017).
Why choose monoclonal antibodies for your experiment
Monoclonal antibodies are particularly useful when you want to ensure that the antibody binds to a specific region of your protein of interest. For example, if you are studying a protein that may bind a ligand during your experiment, then you’d want to make sure that you choose an antibody that recognizes an epitope far from the ligand binding site (Mould et al., 2016). If your antibody recognizes a part of your protein that may be masked while the ligand is bound, then you’ll get a false negative signal - the antibody can’t recognize the protein even though it is present in your sample.
Additionally, there is a lower likelihood that monoclonal antibodies will exhibit off-target binding to unintended proteins. This is because monoclonal antibodies recognize a single epitope, so it’s less likely that this amino acid sequence will be shared across other proteins. Conversely, polyclonal antibodies - which are a heterogenous mixture of antibodies that recognize many different epitopes on the same protein - are more likely to have off-target effects. This is because there are more chances for these antibodies to recognize epitopes that are also present in other proteins.
Because of the specificity of monoclonal antibodies, they also lead to more reproducible experimental results. When you are originally selecting a hybridoma, you can choose one that produces high-affinity/high-avidity antibodies. The same hybridoma is then used to produce all lots of the antibody, meaning that the antibodies are largely identical between lots.
Disadvantages of monoclonal antibodies
As mentioned above, genetic drift is one concern when using hybridomas to produce monoclonal antibodies. Genetic drift refers to changes in the nucleic acid sequence of the antibody-encoding genes over time as the hybridoma cells divide. These changes can alter the antibody that is actually produced by the hybridoma, meaning that there will be changes between lots over time. Specifically, the antigen-binding sites of the antibody - the paratopes - may change and impact the specificity and avidity of the antibodies produced by the hybridoma. Any genetic drive can be identified by sequencing the heavy and light chains. To circumvent any possible genetic drift, scientists can freeze hybridoma cells before culturing or they can clone the genes from the hybridoma into a plasmid to create a recombinant antibody.
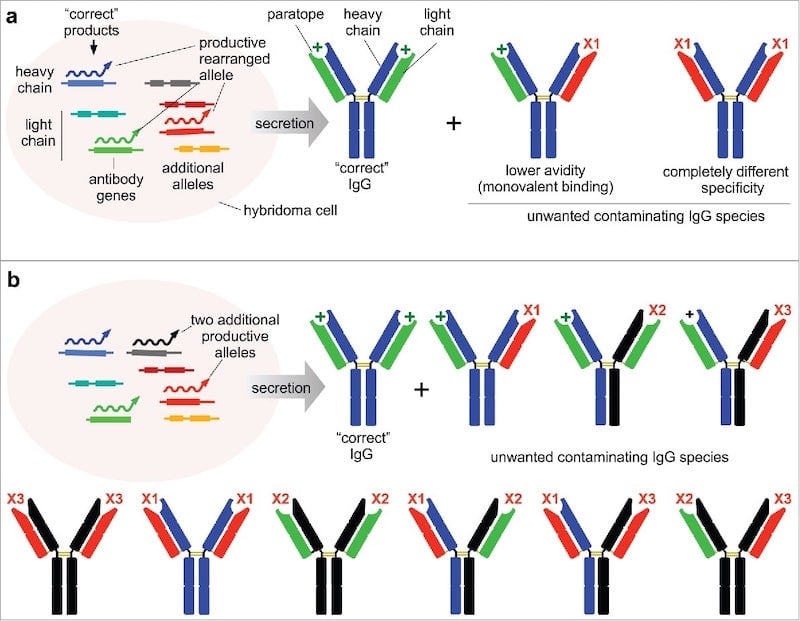 |
| Figure 2: Changes to the heavy chain or light chain genes within a hybridoma impacts the specificity, avidity, and number of different antibodies produced by the hybridoma. Image from Bradbury et al., 2018. |
Another barrier to using monoclonal antibodies in the lab may be their cost. Because of the steps involved in producing and validating monoclonal antibodies, their cost is higher than that of polyclonal antibodies. For example, it takes longer to develop a hybridoma than it does to immunize an animal and recover polyclonal antibodies (Lipman et al., 2005). This is reflected in the antibody’s cost.
Conclusion
Whether you choose to use a monoclonal antibody for your experiment versus other types of antibodies depends on a variety of factors. Understanding the advantages and disadvantages of monoclonal antibodies will help you make the best decision for your experimental needs.
References and resources
References
Bradbury ARM, Trinklein ND, Thie H, Wilkinson IC, Tandon AK, Anderson S, Bladen CL, Jones B, Aldred SF, Bestagno M, Burrone O, Maynard J, Ferrara F, Trimmer JS, Görnemann J, Glanville J, Wolf P, Frenzel A, Wong J, Koh XY, Eng H-Y, Lane D, Lefranc M-P, Clark M, Dübel S (2018) When monoclonal antibodies are not monospecific: Hybridomas frequently express additional functional variable regions. mAbs 10:539–546. https://doi.org/10.1080/19420862.2018.1445456
Holzlöhner P, Hanack K (2017) Generation of Murine Monoclonal Antibodies by Hybridoma Technology. JoVE. https://doi.org/10.3791/54832
Lipman NS, Jackson LR, Trudel LJ, Weis-Garcia F (2005) Monoclonal Versus Polyclonal Antibodies: Distinguishing Characteristics, Applications, and Information Resources. ILAR Journal 46:258–268. https://doi.org/10.1093/ilar.46.3.258
Mould AP, Askari JA, Byron A, Takada Y, Jowitt TA, Humphries MJ (2016) Ligand-induced Epitope Masking. Journal of Biological Chemistry 291:20993–21007. https://doi.org/10.1074/jbc.m116.736942
National Research Council (US) Committee on Methods of Producing Monoclonal Antibodies. Monoclonal Antibody Production. Washington (DC): National Academies Press (US); 1999. 1, Generation of Hybridomas: Permanent Cell Lines Secreting Monoclonal Antibodies. https://www.ncbi.nlm.nih.gov/books/NBK100203/
Additional resource on the Addgene blog
- Learn more about research applications for antibodies in Intro to Antibodies
- Read about the Developmental Studies Hybridoma Bank, a repository for hybridomas
- Learn about monoclonal antibodies for immunotherapy
Resource on Addgene.org
- Check out Addgene’s antibody plasmid collection
- Read the latest on Addgene's new antibody project
Topics: Antibodies







Leave a Comment