You’ve designed the perfect experiment – controls, conditions, and everything in between – now all you need are some of your favorite proteins purified to carry out your plan. With a little thoughtful planning, affinity tags can make protein purification a cinch. Types of affinity tags, the proteins they work best attached to, and the species they are compatible with are all reviewed here.
What are affinity tags?
Affinity tags are used primarily as protein purification tools (and can have secondary functions as detection aids.) When a single protein is expressed, with the intent of purification, it will be expressed along with all other cellular proteins within the expression system (bacteria, yeast, mammalian, etc.). To individually isolate a protein, there must be a special feature about that protein that no other protein in the expression system has. This is where affinity tags come in – they are peptides which do not naturally exist in most expression systems that can be added to the N or C terminus of your protein of interest. Many of these tags also have commercially available antibodies, resins, beads, and other purification aids readily available. Choosing the best tag for your experiment will likely depend on:
- The protein you are trying to purify
- The expression system
- If you want the tag to remain on your protein for downstream applications
Below, we describe a few of the most common tags available.
Maltose binding protein
Maltose binding protein (MBP) is a 45 kDa tag which can be purified (along with its fusion protein) with amylose resin. The MBP tag, although large, can actually increase expression and solubility of the tagged protein (Fox and Waugh, 2002). The tag can also be proteolytically cleaved after purification, so as not to perturb protein function for downstream applications. Tag cleavage must be performed after primary purification and requires a second clean up step to remove the cleaved tag from solution. MBP antibodies are available for detection and/or purification of MBP-tagged proteins. The antibody is an alternative to amylose resin and also provides a visual detection mechanism via applications such as western blot and immunofluorescence.
GST
Glutathione S-Transferase (GST) is a 26 kDa tag which can be isolated with glutathione resin. Like MBP, it can be removed via protease cleavage at sites immediately following the tag. Unlike MBP, the GST tag can be cleaved off from the fusion protein while it is still bound to the glutathione resin, eliminating the need for an extra clean up step of the tag – a handy feature. If you decide not to cleave the GST tag after/during purification, be aware that the tag dimerizes in solution, which can affect downstream applications. Also, purifying GST by affinity chromatography requires proper folding of the tag, which prevents insoluble fusion proteins from being purified by GST (maybe try MBP if you are having solubility issues!).
Need a GST antibody? Addgene has you covered!
His
His tags, often called polyhistidine tags, typically include six consecutive His residues (or more). Histidine bonds with immobilized metal ions (Nickel, Cobalt, Copper, etc.), allowing His fusion proteins to be purified by single step metal affinity chromatography. Purification of His fusion proteins from E. coli is generally more straightforward and of a higher purity than mammalian purifications due to the presence of more naturally occurring histidine-rich proteins in mammals. Mammalian purification is still possible but requires optimization and often more stringent washing. Nonetheless, this tag can be purified with several different resin types and is a fairly small tag that is unlikely to perturb protein function. This makes it amenable to being left on your protein of interest, especially if downstream antibody detection is desired… and yes, we have an antibody for that!
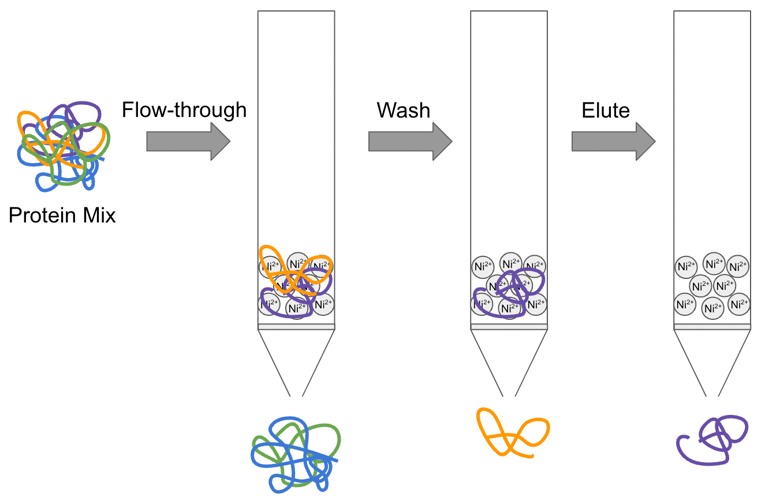
Fig. 1 - Diagram of His-tagged protein purification work flow by nickel column. |
Affinity tags vs. other tags
There are many different types of tags, including epitope tags (FLAG, Myc, etc.) and fluorescent protein tags (GFP, mCherry, etc.). Fluorescent tags are primarily used for direct visualization while epitope tags are used for both direct and indirect visualization. Some of these tags can also be used for protein purification, just as affinity tags can sometimes be repurposed for visualization. However, due to the nature of the size, structure, and other qualities of each tag class, they are generally best suited for their primary application.
Ready to start tagging? We are here to help with vectors, protocols, antibodies, and more!
Resources and References
References
Coligan, J. E., Dunn, B. M., Speicher, D. W., & Wingfield, P. T. (Eds.). (2001). Current Protocols in Protein Science. John Wiley & Sons, Inc. https://doi.org/10.1002/0471140864
Fox, J. D., & Waugh, D. S. (2002). Maltose-Binding Protein as a Solubility Enhancer. In P. E. Vaillancourt, E. coli Gene Expression Protocols (Vol. 205, pp. 99–118). Humana Press. https://doi.org/10.1385/1-59259-301-1:99
Resources on Addgene.org
Resources on the Addgene blog
Topics: Antibodies





Leave a Comment