In April 2022, physicians in Scotland began noticing unusual cases of acute hepatitis in the pediatric population. Once ~400 cases had amassed in places around the world, hypotheses began to develop that this illness was caused by either SARS-CoV-2, Adenovirus type 41 (AdV-41), or Adenovirus-Associated Virus type 2 (AAV2) – or some unprecedented combination of the three (Servellita et al., 2023).
Normally when children have acute viral hepatitis it is caused by the Hepatitis A Virus, for which a vaccine is available. However, here it appears that the culprit for these unusual cases was either 1) a physiological effect of SARS-CoV-2 toward destabilizing one’s natural immune response to Adenovirus (and/or to its partner, AAV) or 2) a sociological effect of the COVID-19 lockdowns causing a lack of exposure, a lack of immunity, and then finally a synchronized exposure under these conditions once the measures were lifted (Servellita et al., 2023).
This anecdote goes toward saying that host-virus interactions are an important part of the biology of adenoviruses (AdVs). AdVs are ubiquitous (Schmitz et al., 1983), yet rarely cause severe disease in humans (and no vaccines are available on the commercial market). Although host-virus interactions likely seem important for any virus, we will see that they are very central determinants of how AdVs behave, and in fact, of how adenoviral vectors work.
What are adenoviruses?
Adenoviral vectors are commonly derived from the mastadenovirus members (mammalian-infecting members) of the Adenoviridae family, which is a very diverse family of viruses with broad host range and tissue tropism (Borkenhagen et al., 2019). AdVs are non-enveloped double-stranded DNA viruses, with a linear genome between 26–45 kb in length. Human adenovirus type 5 (hAdV-C5) can be considered the prototype, although many viruses infecting humans (from seven species, designated as letters A–G) exist. The life cycle of AdVs is dependent on modulation of the host cell’s cell cycle; inhibition of the innate immune system; and reconfiguring of the nuclear compartment, gene expression, and protein translation. Its genome consists of “early” (E) and “late” genes, and the transition between early and late infection (and therefore between early and late gene transcription) is demarcated by viral genome replication (Harrach, 2014).
Given that the early genes encode proteins essential to initiating the whole process – such as the viral DNA polymerase – it makes sense that vectorization strategies include creating an AdV transfer plasmid with the E1 and E3 (Early 1 and Early 3) genes deleted. These genes’ functions are rescued by helper plasmids used during production and/or by genes present in the HEK293T cell line used (which have fragments of the AdV genome in them, including the E1 region). The space in the viral particle’s capacity that is made available by this deletion from the genomic plasmid can be used to house a recombinant sequence (transgene) instead.
Although the late genes are usually present in the transfer plasmid for the majority of adenoviral vector systems used in research, they are only expressed in the producer cells where there is E1 and E3 gene complementation. In target cells that are infected by the vector, only the transgene will be expressed, since the early genes are absent, and the late genes (and their structural proteins, which would encapsidate new virions) are not expressed. Note that replication-competent AdV vectors also exist and are used in certain contexts (Bauzon et al., 2003).
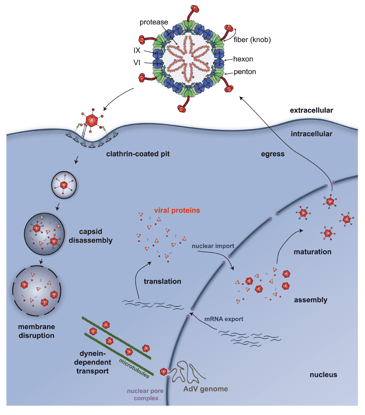
Figure 1: The adenovirus life cycle. Image reused from Kremer and Nemerow 2015 under CC-BY license.
The promise of adenoviral vectors
This brings us to the final topic, which is that natural AdVs, replication-competent viruses with a recombinant genome, and adenoviral vectors all have a genomic capacity that is much higher than other viral vectors (tens of kilobases; compared to only 4.7 kb for AAV, 9 kb for retroviruses). The tradeoff is that adenoviral vector expression is very short-lived. It is worth noting that it is possible to design a vector that achieves persistent transgene expression in certain cell types (Tatsis et al., 2007), potentially because some of the wild-type viruses can persist as a latent infection in certain tissue reservoirs (Pereira, 1972).
However, the majority of adenoviral vector expression is transient due to the fact that AdV surface proteins (especially fiber’s knob domain, and hexon), as well as other intracellularly-exposed proteins and downstream cellular events, all recruit strong innate and adaptive immune responses that inactivate viral genomes or otherwise clear the virus from the cell population (Kafri et al., 1998). In fact, adenovirus gets its name because it was first isolated from human adenoid tissue and rapidly caused the death of adenoid cells in vitro (Rowe et al., 1953). While overt death of the targeted cells is less likely for adenoviral vectors – namely due to the removal of certain adenoviral genes that apparently contribute to virulence (Tollefson et al., 1996) – cell death has been reported when using vectors, and immune inactivation of the vector can still occur even in the case of no previous immunity existing for that adenoviral vector (Hofmann et al., 1999).
The ability of the adenovirus particle to activate the immune system, and of the infection to sometimes result in cell death, has led to interest in using adenoviral vectors for vaccine delivery and for oncolytic virus in addition to standard uses in gene therapy and in basic research, like other viral vectors, though significant challenges remain (Wold & Toth, 2013).
While the pre-existing toolbox for adenoviral vectors in Addgene’s repository is smaller than for other vector types, we hope that this introduction gives a sense of what AdVs are about – and we hope that it inspires further use of existing vectors, as well as research into the development of new adenoviral vectors.
This post was written by Addgenie Brian O'Neill.
References and resources
References
Bauzon, M., Castro, D., Karr, M., Hawkins, L. K., & Hermiston, T. W. (2003). Multigene expression from a replicating adenovirus using native viral promoters. Mol Ther, 7(4), 526–534. https:/doi.org/10.1016/S1525-0016(03)00023-6. PMID: 12727116.
Borkenhagen, L. K., Fieldhouse, J. K., Seto, D., & Gray, G. C. (2019). Are adenoviruses zoonotic? A systematic review of the evidence. Emerg Microbes Infect, 8(1), 1679–1687. https://doi.org/10.1080/22221751.2019.1690953. PMID: 31749409.
Harrach, B. (2014). Adenoviruses: General Features. In Reference Module in Biomedical Sciences. Elsevier. https://doi.org/10.1016/B978-0-12-801238-3.02523-X.
Hofmann, C., Löser, P., Cichon, G., Arnold, W., Both, G. W., & Strauss, M. (1999). Ovine Adenovirus Vectors Overcome Preexisting Humoral Immunity against Human Adenoviruses In Vivo. J Virol, 73(8), 6930–6936. https://doi.org/10.1128/JVI.73.8.6930-6936.1999. PMID: 10400791.
Kafri, T., Morgan, D., Krahl, T., Sarvetnick, N., Sherman, L., & Verma, I. (1998). Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: Implications for gene therapy. Proc Natl Acad Sci U S A, 95(19), 11377. https://doi.org/10.1073/PNAS.95.19.11377. PMID: 9736744.
Kremer, E. J., & Nemerow, G. R. (2015). Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex. PLoS Pathog, 11(6), e1004821. https://doi.org/10.1371/journal.ppat.1004821. PMID: 26042599.
Pereira, H. G. (1972). Persistent infection by adenoviruses. J Clin Pathol Suppl (R Coll Pathol)., 6, 39. https://doi.org/10.1136/jcp.s3-6.1.39. PMID: 4376152.
Rowe, W. P., Huebner, R. J., Gilmore, L. K., Parrott, R. H., & Ward, T. G. (1953). Isolation of a Cytopathogenic Agent from Human Adenoids Undergoing Spontaneous Degeneration in Tissue Culture. Proc Soc Exp Biol Med. 84(3), 570–573. https://doi.org/10.3181/00379727-84-20714. PMID: 13134217.
Schmitz, H., Wigand, R., & Heinrich, W. (1983). Worldwide epidemiology of human adenovirus infections. Am J Epidemiol, 117(4), 455–466. https://doi.org/10.1093/oxfordjournals.aje.a113563. PMID: 6301263.
Servellita, V., Sotomayor Gonzalez, A., Lamson, D. M., Foresythe, A., Huh, H. J., Bazinet, A. L., Bergman, N. H., Bull, R. L., Garcia, K. Y., Goodrich, J. S., Lovett, S. P., Parker, K., Radune, D., Hatada, A., Pan, C. Y., Rizzo, K., Bertumen, J. B., Morales, C., Oluniyi, P. E., Nguyen, J., … Chiu, C. Y. (2023). Adeno-associated virus type 2 in US children with acute severe hepatitis. Nature, 617(7961), 574–580. https://doi.org/10.1038/s41586-023-05949-1. PMID: 36996871.
Tatsis, N., Fitzgerald, J. C., Reyes-Sandoval, A., Harris-McCoy, K. C., Hensley, S. E., Zhou, D., Lin, S. W., Bian, A., Zhi, Q. X., Iparraguirre, A., Lopez-Camacho, C., Wherry, E. J., & Ertl, H. C. J. (2007). Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood, 110(6), 1916–1923. https://doi.org/10.1182/BLOOD-2007-02-062117. PMID: 17510320.
Tollefson, A. E., Scaria, A., Hermiston, T. W., Ryerse, J. S., Wold, L. J., & Wold, W. S. (1996). The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J Virol, 70(4), 2296. https://doi.org/10.1128/JVI.70.4.2296-2306.1996. PMID: 8642656.
Wold, W. S. M., & Toth, K. (2013). Adenovirus Vectors for Gene Therapy, Vaccination and Cancer Gene Therapy. Curr Gene Ther, 13(6), 421–433. https://doi.org/10.2174/1566523213666131125095046. PMID: 24279313.
More resources on the Addgene blog
Viral Vectors 101: Viral Applications
Topics: Viral Vectors 101, Adenoviral Vectors







Leave a Comment