This guest post was contributed by Sana Khan Khilji, a PhD student at the Max Planck Institute of Colloids and Interfaces.
Here are some tips for cell culture that will hopefully help you keep a well organized lab and contamination free environment for successful experiments:
 To clean air and beyond
To clean air and beyond
Turn on the hood for at least 15 minutes before you start any tissue culture work and make sure that clean air is flowing in. The vent for the in-flowing air is normally visible and you should always ensure nothing is covering it. Turning on the UV light 3-4 times a week for up to half an hour can help keep your hood contamination free, but you should keep in mind that UV light only kills microorganisms that it strikes directly. UV light exposure is also harmful for eyes and skin so keep the light off when the hood is in use. Wipe the hood with a disinfectant (most people use 70% ethanol, but you can also use Incidin wipes) before and after your work.
Keep your contaminants away
Learn more about cell culture contamination in this post from the ATCC
Declutter! Keep the hood clean
Make sure cells don't stay out too long
While you’re preparing things, make sure your cells don’t stay out too long. Cells usually have their comfort zone around 37 °C and 5% CO2 and get agitated with sudden changes in temperature (especially if you have air conditioning on) so leave them in a sterile incubator. This also means that any medium or washing buffer, such as PBS (Phosphate Buffered Saline), that comes in contact with the cells should already be at 37 °C. Most labs have a water bath constantly at 37°C for this purpose.
Learn about the effects of CO2 on sample pH
Cell morphology: Shape and size matter
You should have a light microscope close to the hood so you can view your cells regularly (both before entering the hood and when putting them into the incubator). It also helps to get familiar with the morphology of the cell line you’re working with so that cross-contamination can be identified. Even if some mammalian cells lines have similar morphologies, being familiar with these morphologies and doing a quick check under the light microscope will help you identify bacterial and yeast contaminants.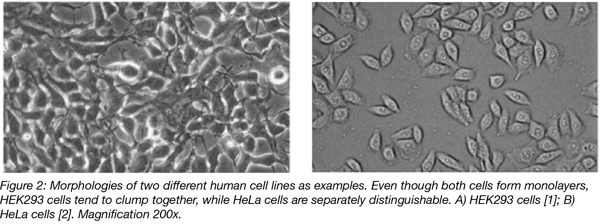
Set up a routine to split cells
There is power in numbers!
Learn how Addgene uses barcodes to track samples
Keep track of passage numbers
Keep an eye on the passage number. Cells change their growth rate and morphology at high passage numbers (>30-40 times), depending on the cell line so old cells should be retired! Conversely, cells younger than the 3rd – 4th passage are too young and should not be used for important transfection experiments right after they’ve been thawed. Additional passages will allow your cells to achieve a consistent growth rate.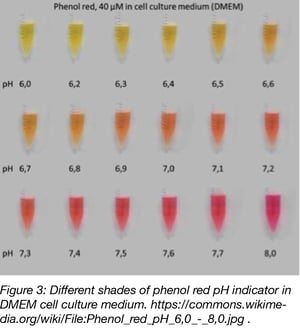
For every minute spent organizing, an hour is earned (Benjamin Franklin)!
It’s good practice to label your plates and/or flasks with your name, date, the cell line you’re using and its passage number. Especially in a lab where multiple people are working on different things, this will help you avoid cross-contamination through accidental mixing and will also help you keep good records of your experiments.
Autoclave is the rave!
Happy culturing!
Many thanks to our guest blogger, Sana Khan Khilji!
 Sana Khan Khilji recently acquired her Master’s degree in Biochemistry from Freie Universität Berlin, Germany. She will soon be joining her PhD lab at the Max Planck Institute of Colloids and Interfaces. Sana is particularly interested in Glycobiology and Immunology. You can find Sana on LinkedIn.
Sana Khan Khilji recently acquired her Master’s degree in Biochemistry from Freie Universität Berlin, Germany. She will soon be joining her PhD lab at the Max Planck Institute of Colloids and Interfaces. Sana is particularly interested in Glycobiology and Immunology. You can find Sana on LinkedIn.
References
1. Yin, R., et al. "Gene expression profiling analysis of bisphenol A-induced perturbation in biological processes in ER-negative HEK293 cells." PLoS One, 2014. 9(6): p. e98635. PubMed PMID: 24901218. PubMed Central PMCID: PMC4047077.
2. Pagliara, Patrizia, et al. "Ostreopsis cf. ovata induces cytoskeletal disorganization, apoptosis, and gene expression disregulation on HeLa cells." Journal of applied phycology27.6 (2015): 2321-2332.
3. Contamination, Ileana S., John P. S., Basic Methods, Human Stem Cell Manual (Second Edition), 2012.
Additional Resources on the Addgene Blog
- The Challenges of Cell Culture
- Cloning Mammalian Cells with the Agarose Method
- Plasmids 101: Mammalian Vectors
Resources on Addgene.org
- Find mammalian plasmids for your research
- Find viral vectors for infecting mammalian cells
Topics: Other, Miscellaneous






Leave a Comment