Optogenetics, the use of light sensitive proteins (opsins) to manipulate cell activity, enables researchers to silence or incite neuronal firing and study subsequent effects on behavior. The system is an especially powerful tool for in vivo behavioral studies because it is non-invasive and offers a high degree of control over time and space.
Zebrafish have become a popular model organism because their larval stage lends itself well to studies of neuroscience. The larvae of zebrafish are translucent and allow for noninvasive live imaging with fluorescent tags and activation of light sensing proteins. Furthermore, during the first 2 weeks of life, larvae already exhibit distinct behaviors such as spontaneous swimming and escape reflex. These traits, coupled with short generation times and high fecundity, make zebrafish ideal for high throughput studies of optogenetics.
Common opsins used in zebrafish
Optogenetics functions through opsins which are light sensitive proteins. Opsins used in optogenetic studies are not native to vertebrates and are commonly introduced in zebrafish through the UAS/Gal4 system for behavioral studies. In neuroscience, opsins are typically ion channels that allow charged particles to cross the cell membrane when exposed to light. Neuronal firing depends on these charged particles and whether they make the inside of the neuron more positive or more negative.
Neuronal stimulation: Depolarization
When the inside of the neuron becomes positive over a certain threshold, it becomes depolarized and will fire a signal. The non-specific cation channel ChR2 and the calcium channel LiGluR both cause ion movement that results in cell depolarization. They therefore function as a neuron “on switch.”
Neuronal inhibition: Hyperpolarization
Conversely, when the inside of the neuron becomes more negative it becomes less likely to fire and is said to be hyperpolarized. The chloride pump NpHR brings in negative chloride ions which hyperpolarize the cell. It therefore functions as a neuron “off switch.”
Because of the differing peak absorption wavelengths and light intensity requirements of different opsins, studies have shown that some channels, such as ChR2 and NpHR, can even be expressed simultaneously and still be individually activated with different lights (Baier et al., 2009).
Zebrafish behaviors
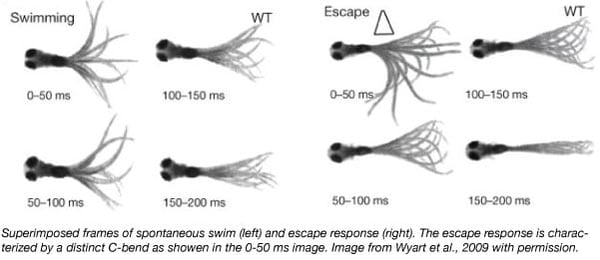
Several behavioral assays can be done on zebrafish at 5 days post fertilization, including escape behavior and spontaneous swimming. When startled by acoustic, visual or tactile stimuli, larval zebrafish react by performing a distinct C-bend to escape possible danger (see figure). Larvae also perform spontaneous slow swimming where they periodically beat their tails left and right in a symmetrical motion to move forward. This behavior is not done in response to any specific stimulus. While the escape response neural pathway is well characterized, there is much left to be discovered of spontaneous swimming.
Zebrafish behavior studies in action: Identifying neurons that trigger spontaneous swimming
Researchers at the Isacoff lab applied optogenetics in zebrafish larvae to identify new neurons that trigger the CPG (central pattern generator) (Wyart et al., 2009). The CPG is a neural circuit involved in locomotion that generates periodic motor commands for rhythmic movements like spontaneous swimming. To do so, the lab expressed the LiGluR opsin in different subsets of spinal cord neurons using the Gal4/UAS system. This created fish lines with different groups of neurons that could be activated with light.
Find plasmids from the Isacoff lab!
They then tested what kind of swimming behavior, spontaneous swim or escape, was elicited when these different neuron groups were activated or “turned on.” In order to distinguish between swimming behaviors, the researchers measured the tail movements of 5 day old fish. The larvae were immobilized in agar at the head with their tails still free, and different regions of the spinal cord were illuminated. By looking at groups of neurons that elicited similar tail movements, they were able to narrow down the possible specific neurons responsible for the response based on the overlap between groups.
They found that photostimulating the Kolmer-Agduhr cells was sufficient to trigger the symmetrical tail movement unique to spontaneous slow forward swimming. Furthermore, only Kolmer-Agduhr cells on one side of the body required photostimulation in order to drive the full symmetrical response.
The function of Kolmer-Agduhr cells had previously been a mystery, but through the control offered by the optogenetics toolbox and the versatility of the zebrafish model organism, the Isacoff lab was able to link these cells to their pivotal role in spontaneous swimming.
References
Arrenberg, Aristides B., Filippo Del Bene, and Herwig Baier. "Optical control of zebrafish behavior with halorhodopsin." Proceedings of the National Academy of Sciences 106.42 (2009): 17968-17973. PubMed PMID: 19805086. PubMed Central PMCID: PMC2764931.
Portugues, Ruben, et al. "Optogenetics in a transparent animal: circuit function in the larval zebrafish." Current opinion in neurobiology 23.1 (2013): 119-126. PubMed PMID: 23246238.
Wyart, Claire, et al. "Optogenetic dissection of a behavioural module in the vertebrate spinal cord." Nature 461.7262 (2009): 407. PubMed PMID: 19759620. PubMed Central PMCID: PMC2770190.
Additional resources on the Addgene blog
- Find all our optogenetics blog posts
- Read blog posts about viral vectors
- Subscribe to the Addgene blog
Resources on Addgene.org
- Learn about our ready-to-use viral service
- Find optogenetics AAVs for your research
- Find all AAVs from the viral service
Topics: Optogenetics, Viral Vectors, Organisms






Leave a Comment