 Mark Howarth’s lab at the University of Oxford is dedicated to generating new tools to manipulate biology based on molecular features found in nature, with the ultimate goal to improve the diagnosis of disease, and cancer in particular. They recently introduced the SpyTag/SpyCatcher system, based on a protein isolated from Streptococcus pyogenes that locks itself together, to produce irreversible protein-peptide interactions. In a study published in Proceedings of the National Academy of Sciences in March, he and his colleagues took another important step forward by dissecting that S. pyogenes protein into three parts. Their efforts yielded a protein, which they call SpyLigase (Spy comes from the “S” in Streptococcus and the “py” in pyogenes), capable of locking two peptide tags together.
Mark Howarth’s lab at the University of Oxford is dedicated to generating new tools to manipulate biology based on molecular features found in nature, with the ultimate goal to improve the diagnosis of disease, and cancer in particular. They recently introduced the SpyTag/SpyCatcher system, based on a protein isolated from Streptococcus pyogenes that locks itself together, to produce irreversible protein-peptide interactions. In a study published in Proceedings of the National Academy of Sciences in March, he and his colleagues took another important step forward by dissecting that S. pyogenes protein into three parts. Their efforts yielded a protein, which they call SpyLigase (Spy comes from the “S” in Streptococcus and the “py” in pyogenes), capable of locking two peptide tags together.
SpyLigase overcomes limitations in the use of peptide tags, which often form only weak and reversible bonds. Howarth’s team has already demonstrated in their PNAS paper that SpyLigase can be used to link affibodies or antibodies against common tumor markers to subsequently capture cancerous cells expressing low levels of tumor antigen. I asked Howarth to tell us more about SpyLigase, its development, and its potential uses.
Developing a new tool
Addgene: Where did you get the idea to develop SpyLigase?
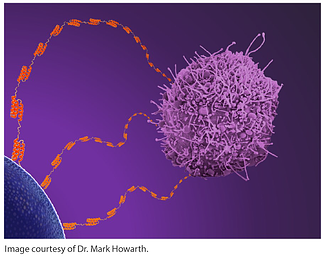
Howarth: It came from our work in developing the SpyTag/SpyCatcher system, to be able to grab on to proteins irreversibly in a genetically encoded way. SpyTag is nice and small, just 13 amino acids, but SpyCatcher is a protein. You can fuse SpyCatcher onto things, but even better if one could just join one peptide tag to another peptide tag.
Addgene: What is the advantage of peptide tags for building larger protein assemblies?
Howarth: Peptide tags can be very generic; you should be able to use the same peptide tag for all sorts of different protein systems and they shouldn’t get in the way much. Particularly we wanted to be able to build assemblies through covalent bonds so that the assemblies wouldn’t fall apart, even after days or if there are forces acting on them. For the application we were looking at, magnetic isolation of circulating tumor cells, we found that the binding stability really mattered for efficient capture. We wanted to be able to build a highly multivalent assembly connected by covalent bonds, so there were no weak links in the chain.
Addgene: What about the Streptococcus pyogenes protein domain made it ideal for this purpose? What led you to it?
Howarth: Our collaborator Uli Schwarz-Linek at St. Andrews discovered the domain. It is small, highly stable and has this amazing chemistry locking the domain together - no one knows what for. Many other Gram-positive bacteria have this chemistry in their surface proteins. It is best studied in the pili that they use to anchor on to host cells.
Addgene: Were there any major hurdles along the way?
Howarth: Just the usual blood, sweat and tears. (Thanks to our healthy blood donor for keeping going while we worked out the cell capture system.)
Cancer and other applications
Addgene: What does this technology mean in the context of cancer?
Howarth: The capture of circulating tumor cells (CTCs) is going to have a big impact on our understanding and treatment of cancer. It is clear that one needs to capture a diverse range of CTCs to cope with the variation in cancer, not just the CTCs with high marker expression level. Labs have been trying different approaches to improve CTC capture, but I hope that our antibody tentacles may have an impact on this challenge either for magnetic capture or for capture using microfluidic devices.
Addgene: What other uses do you foresee for this tool?
Howarth: Polyproteins are useful for mechanical study of proteins by atomic force microscopy (AFM). Maybe polyantibodies could help in the capture of other rare cell types, like stem cells or cells infected with malaria or other parasites.
Addgene: Do you have any particular tips or tricks for those wishing to purchase and use the SpyLigase system for the first time?
Howarth: The Proceedings of the National Academy of Sciences paper [published online on March 17] should cover it.
Addgene: OK, thanks for your time, and thanks for depositing your plasmids with Addgene.
Howarth: We submit all our plasmids that may well be of use to others to Addgene. I am a big fan; thanks for the T-shirt!
References:
SpyLigase peptide-peptide ligation polymerizes affibodies to enhance magnetic cancer cell capture. Fierer et al (Proc Natl Acad Sci U S A. 2014 Mar 17. PubMed)
Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Zakeri et al (Proc Natl Acad Sci U S A. 2012 Mar 20;109(12):E690-7. Epub 2012 Feb 24. PubMed)
Topics: Plasmid Tags, Plasmids






Leave a Comment