This post was contributed by guest blogger Daria Shcherbakova, a faculty member at Albert Einstein College of Medicine.
Several sets of near-infrared fluorescent proteins (NIR FPs) and biosensors have been created recently. As developers of many of these probes, we decided to write this quick guide to help researchers choose the probe for their needs. You’ll find basic information on NIR FPs, their properties and applications. At the end, there is a Table that summarizes the properties of state-of-the-art probes, many of which you can find at Addgene.
Biliverdin chromophore
Near-infrared fluorescent proteins (NIR FPs) were derived from natural photoreceptors incorporating biliverdin IXα (BV) autocatalytically. Fortunately for probe developers and users, BV is present in eukaryotic cells as an intermediate of heme metabolism (Shemetov et al, 2017). Modern state-of-the-art NIR FPs can be used similar to GFP-like probes by supplying a single gene to cells. Still in bacteria, which do not produce BV, an additional gene coding for heme oxygenase should be supplied (Baloban et al., 2017).
It is important to consider effective brightness of NIR FPs in cells, in addition to their molecular brightness (a product of the fluorescence quantum yield and the extinction coefficient) (Shcherbakova et al., 2015). The effective brightness reflects the concentration of BV-containing molecules in a cell and depends on the efficiency of BV incorporation by an apoprotein and protein expression level. State-of-the-art FPs (listed in Table 1) were optimized for efficient binding of endogenous BV.
When imaging NIR FPs, consider using light sources that produce enough light intensities in NIR. Usually, mercury lamps are suboptimal, metal halide lamps are better, and xenon lamps are the best for working in this spectral range. You can also check that no filters and/or cold mirrors block the NIR light coming from your source and to your camera.
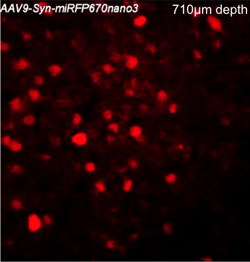 |
|
Two-photon image of miRFP670nano3-expressing neurons in the neocortex of a living mouse. Image courtesy of Axel Nimmerjahn (Salk Institute for Biological Studies)
|
Multiple uses: From multiplexing to multiscaling
NIR-shifted spectrum is perfect for use in spectral multiplexing (Shcherbakova et al., 2018). NIR FPs can be combined with blue-light controlled optogenetics, such as channelrhodopsins and tools based on light-oxygen-voltage (LOV) domain. They can also be used in crosstalk-free three-color imaging with green and red GFP-like FPs (Shcherbakova et al., 2016), as well as in four-color imaging (cyan, yellow, red, and near-infrared) (Qian et al., 2019).
NIR FPs are useful in vivo probes, due to low autofluorescence, low scattering, and deep penetration of red-shifted light. The same NIR FPs and biosensors can be imaged at spatial scales from sub-cellular to whole-body in small animals, such as mice (Matlashov et al., 2020).
Enhanced miRFPs are monomeric and bright
Recent series of enhanced monomeric NIR FPs named miRFPs (monomeric infrared FPs) (Matlashov et al., 2020) derived from bacterial phytochrome (BphPs) photoreceptors have higher apparent cellular brightness than previously reported widely used dimeric iRFPs (Table 1). In general, the monomeric state of a probe leads to less interference with the oligomeric state of a fusion partner. Therefore, we recommend using miRFPs and their enhanced versions named emiRFPs in your applications.
There is a whole set of spectrally distinct (e)miRFPs available. The numbers in (e)miRFP names correspond to the maxima of their emission spectra. These probes can be combined in two-color and multicolor NIR imaging.
Small miRFPnano
A separate class of NIR FPs, named miRFPnanos, were derived from cyanobacteriochrome (CBCR) photoreceptors (Oliinyk et al., 2019). They were engineered to incorporate BV. Their advantages are their small size (17 kDa for miRFPnanos, 35 kDa for miRFPs, and 27 kDa for EGFP) and a possibility to be used as internal tags due to the proximity of the N- and C-termini. Latest miRFPnanos9 do not yield to (m)iRFPs in cellular and molecular brightness (Table 1).
NIR reporters and biosensors
NIR reporters can tell us about cellular events via fluorescence changes occurring due to protein synthesis, degradation, or reconstitution (reviewed in Shcherbakova et al., 2018). For detection of protein-protein interactions, we recommend using NIR split reporters based on miRFPs (Shcherbakova et al., 2016), since they have lower background and higher contrast than previously developed NIR split probes (Tchekanda et al., 2014, Filonov et al., 2013).
FRET biosensors were developed based on miRFP760-miRFP720 FRET pair, including Rac1 biosensor (Shcherbakova et al., 2018) and iGECI calcium indicator (Shemetov et al., 2021). In addition to FRET, intensiometric calcium indicators NIR-GECOs were developed based on mIFP6. Learn more about the characteristics, performance, and applications of near-infrared calcium indicators in this recent review (Shcherbakova et al., 2021).
Table 1: Properties of modern NIR FPs
| NIR FP |
Ex, nm |
Em, nm |
ECa, M-1cm-1 |
QYb, %
|
Molecular brightness vs. iRFP713, % |
Oligomeric state |
Photo-stabilityc, t1/2, s |
pKa |
Brightness in HeLa cells vs. iRFP713, % d |
Ref |
|
|
BphP-based FPs |
miRFP670 https://www.addgene.org/79987/ |
642 | 670 | 87,400 | 14.0 | 198 | Monomer | 155 | 4.5 | 72 | 5 |
|
miRFP703 https://www.addgene.org/79988/ |
674 | 703 | 90,900 | 8.6 | 127 | Monomer | 394 | 4.5 | 37 | ||
|
miRFP709 https://www.addgene.org/79989/ |
683 | 709 | 78,400 | 5.4 | 69 | Monomer | 192 | 4.5 | 30 | ||
|
mIFP https://www.addgene.org/54620/ |
683 | 705 | 82,000 | 8.4 | 112 | Monomer | 54 | 4.5 | 15 | 15 | |
|
miRFP https://www.addgene.org/108409/ |
674 | 703 | 92,400 | 9.7 | 145 | Monomer | ND | 4.3 | ND | 16 | |
|
IFP2.0 e https://www.addgene.org/54785/ |
688 | 709 | 98,000 | 8.1 | 128 | Dimer | 108 | 4.5 | 8 | 17 | |
|
iRFP670 https://www.addgene.org/45457/ |
643 | 670 | 114,000 | 12.2 | 225 | Dimer | 290 | 4.0 | 119 | 18 | |
|
iRFP682 https://www.addgene.org/45459/ |
663 | 682 | 90,000 | 11.1 | 162 | Dimer | 490 | 4.5 | 105 | ||
|
iRFP702 https://www.addgene.org/45456/ |
673 | 702 | 93,000 | 8.2 | 124 | Dimer | 630 | 4.5 | 61 | ||
|
iRFP713 https://www.addgene.org/31857/ |
690 | 713 | 98,000 | 6.3 | 100 | Dimer | 960 | 4.5 | 100 | 19 | |
|
iRFP720 https://www.addgene.org/45461/ |
702 | 720 | 96,000 | 6.0 | 93 | Dimer | 490 | 4.5 | 110 | 18 | |
|
emiRFP670 https://www.addgene.org/136556/ |
642 | 670 | 87,400 | 14.0 | 198 | Monomer | 450 | 4.5 | 136 | 7 | |
|
miRFP680 https://www.addgene.org/136557/ |
661 | 680 | 64,000 | 14.5 | 221 | Monomer | 980 | 4.5 | 182 | ||
|
emiRFP703 https://www.addgene.org/136558/ |
674 | 703 | 90,900 | 8.6 | 127 | Monomer | 700 | 4.5 | 90 | ||
|
miRFP713 https://www.addgene.org/136559/ |
690 | 713 | 99,000 | 7.0 | 122 | Monomer | 980 | 4.5 | 110 | ||
|
miRFP720 https://www.addgene.org/136560/ |
702 | 720 | 98,000 | 6.1 | 97 | Monomer | 510 | 4.5 | 117 | 12 | |
| CBCR-based FPs |
miRFP670nano https://www.addgene.org/127443/ |
645 | 670 | 95,000 | 10.8 | 166 | Monomer |
545 | 3.7 | 62 | 8 |
| miRFP670nano3 | 645 | 670 | 129,000 | 18.5 | 387 | Monomer | 675 | 4.2 | 250 | 9 |
|
|
miRFP704nano |
680 | 704 | 93,000 | 9.9 | 149 | Monomer | 1265 | 4.1 | 81 | ||
|
miRFP718nano |
690 | 718 | 79,000 | 5.6 | 72 | Monomer | 885 | 3.8 | 33 |
State-of-the-art NIR FPs, which combine high cellular brightness and monomeric state, are highlighted in blue. aExtinction coefficient. bQuantum yield. cDetermined in mammalian cells. dDetermined as effective NIR fluorescence in transiently transfected live HeLa cells with no supply of exogenous BV and after normalization to fluorescence of co-transfected EGFP. e Originally reported as a monomer16, IFP2.0 was later found to be a dimer3,15. ND-not determined in parallel assays with other FPs.
We hope that this post helps you in getting to know the state-of-the-art NIR fluorescent probes and in choosing the perfect one for your experiment!
 Thank you to our guest blogger!
Thank you to our guest blogger!
Daria Shcherbakova is a faculty member at Albert Einstein College of Medicine. Her expertise is development and applications of fluorescent proteins, genetically encoded biosensors, and optogenetic tools. Follow her recent research @daria_gift_shch.
References and resources
References
- Shemetov, A.A., Oliinyk, O.S. & Verkhusha, V.V. How to Increase Brightness of Near-Infrared Fluorescent Proteins in Mammalian Cells. Cell Chem Biol 24, 758-766 e753 (2017).
- Baloban, M. et al. Designing brighter near-infrared fluorescent proteins: insights from structural and biochemical studies. Chem Science 8, 4546-4557 (2017).
- Shcherbakova, D.M., Baloban, M. & Verkhusha, V.V. Near-infrared fluorescent proteins engineered from bacterial phytochromes. Curr Opin Chem Biol 27, 52-63 (2015).
- Shcherbakova, D.M., Stepanenko, O.V., Turoverov, K.K. & Verkhusha, V.V. Near-Infrared Fluorescent Proteins: Multiplexing and Optogenetics across Scales. Trends Biotechnol 36, 1230-1243 (2018).
- Shcherbakova, D.M. et al. Bright monomeric near-infrared fluorescent proteins as tags and biosensors for multiscale imaging. Nat Commun 7, 12405 (2016).
- Qian, Y. et al. A genetically encoded near-infrared fluorescent calcium ion indicator. Nat Methods 16, 171-174 (2019).
- Matlashov, M.E. et al. A set of monomeric near-infrared fluorescent proteins for multicolor imaging across scales. Nat Commun 11, 239 (2020).
- Oliinyk, O.S., Shemetov, A.A., Pletnev, S., Shcherbakova, D.M. & Verkhusha, V.V. Smallest near-infrared fluorescent protein evolved from cyanobacteriochrome as versatile tag for spectral multiplexing. Nat Commun 10, 279 (2019).
- Oliinyk, O.S. et al. Single-domain near-infrared proteins provide a scaffold for antigen-dependent fluorescent nanobodies. Nat Methods submitted (2021).
- Tchekanda, E., Sivanesan, D. & Michnick, S.W. An infrared reporter to detect spatiotemporal dynamics of protein-protein interactions. Nat Methods 11, 641-644 (2014).
- Filonov, G.S. & Verkhusha, V.V. A near-infrared BiFC reporter for in vivo imaging of protein-protein interactions. Cell Chem Biol 20, 1078-1086 (2013).
- Shcherbakova, D.M., Cox Cammer, N., Huisman, T.M., Verkhusha, V.V. & Hodgson, L. Direct multiplex imaging and optogenetics of Rho GTPases enabled by near-infrared FRET. Nat Chem Biol 14, 591-600 (2018).
- Shemetov, A.A. et al. A near-infrared genetically encoded calcium indicator for in vivo imaging. Nat Biotechnol 39, 368-377 (2021).
- Shcherbakova, D.M. Near-infrared and far-red genetically encoded indicators of neuronal activity. J Neurosci Methods 362, 109314 (2021).
- Yu, D. et al. A naturally monomeric infrared fluorescent protein for protein labeling in vivo. Nat Methods 12, 763-765 (2015).
- Piatkevich, K.D. et al. A robotic multidimensional directed evolution approach applied to fluorescent voltage reporters. Nat Chem Biol 14, 352-360 (2018).
- Yu, D. et al. An improved monomeric infrared fluorescent protein for neuronal and tumour brain imaging. Nat Commun 5, 3626 (2014).
- Shcherbakova, D.M. & Verkhusha, V.V. Near-infrared fluorescent proteins for multicolor in vivo imaging. Nat Methods 10, 751-754 (2013).
- Filonov, G.S. et al. Bright and stable near-infrared fluorescent protein for in vivo imaging. Nat Biotechnol 29, 757-761 (2011).
Topics: Fluorescent Proteins, Fluorescent Imaging





Leave a Comment