Not all plasmid preps are the same. Before purifying a plasmid from a bacterial culture, it is important to consider your experiment. It will dictate the amount of DNA you need, and at which level of purity. Based on these premises we can classify a plasmid preparation in 3 different ways:
- Transformation grade DNA
- Cloning grade DNA
- Transfection grade DNA
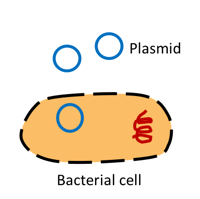 |
| Figure 1: Transformation introduces transformation grade plasmids into a bacterial cell. |
Transformation grade DNA
Transformation is the process of inserting exogenous DNA in a microorganism. The amount of plasmid DNA that is necessary for bacterial transformation of chemically and electrocompetent cells is minimal (picogram range). It can be extracted from small bacterial cultures (i.e. 2-3 ml) with or without commercially available kits and DNA for transformation doesn’t need to be as pure as for other uses.
Cloning grade DNA
Molecular cloning includes a set of techniques that are used to insert recombinant DNA from prokaryotes or eukaryotes into a synthetic vector that can have a plasmid or viral origin. The type of cloning approach you choose ultimately will influence the quantity of DNA that is required and, as a consequence, also dictate the required sizes of the bacterial cultures and commercial kits you will adopt for the extraction. Restriction enzyme based approaches can require several hundreds of nanograms or micrograms of starting material; Gibson assembly or Golden gate assembly use a more direct approach, allowing scientists to clone from a smaller amount of DNA (ng range).
To allow efficient and flawless cloning it is important that the preparation is pure from other nucleic acids (genomic DNA and RNA), proteins and chemical contaminants that have been used during the purification procedure. Good indicators of DNA purity are the absorbance measurements at 230, 260, and 280 nm. The absorbance ratios measured at 260 and 280 nm should fall ~1.8 - 2 and give scientists an indication of purity versus protein contaminants. The 260 and 230 nm ratios should fall ~2 - 2.2, and provide an indication of purity versus chaotropic agents as guanidine thiocyanate and guanidine hydrochloride used during plasmid extraction.
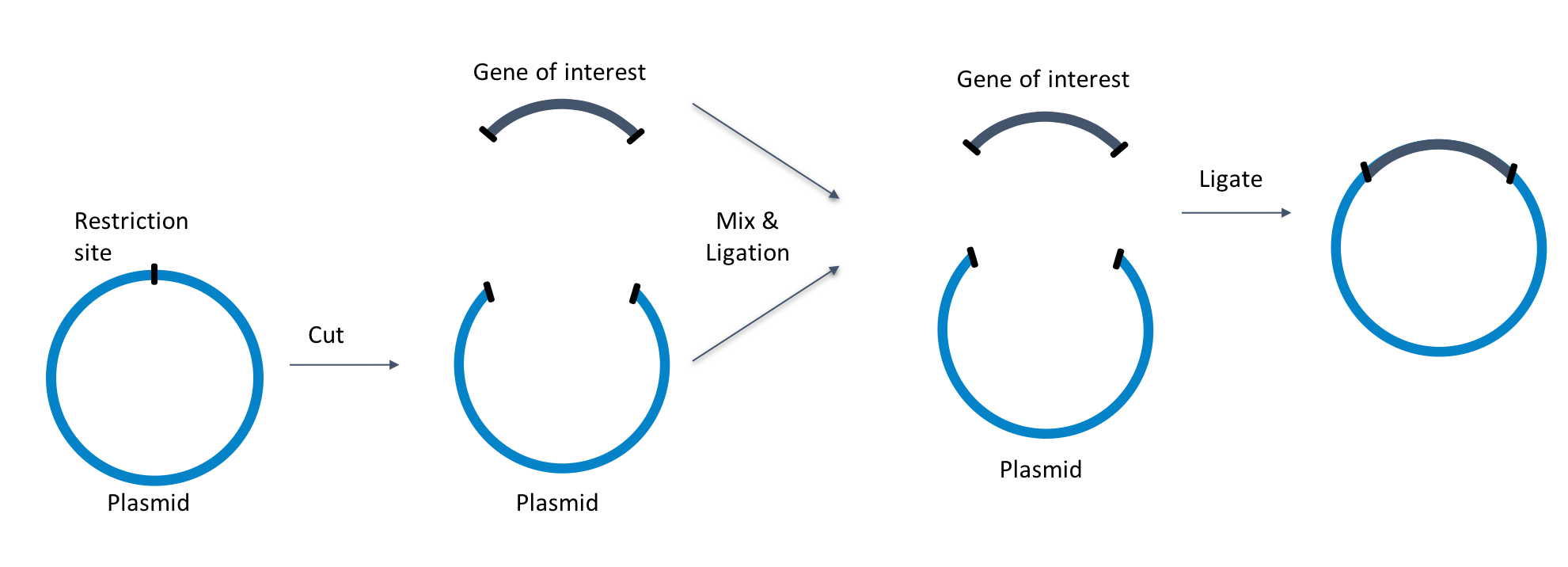 |
| Figure 2: Use cloning grade DNA for direct cloning of your gene of interest into a plasmid backbone. |
Transfection grade DNA
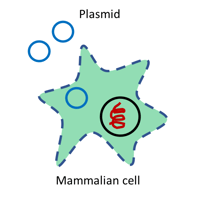 |
| Figure 3: Need to introduce plasmids into mammalian cells? Use transfection grade DNA. |
Transfection is the process of inserting an external plasmid in mammalian cells and it will likely require micrograms of plasmid DNA for each reaction. As a consequence, the preparations for this technique must be larger scale, to allow the extraction of enough plasmid to last for multiple experiments.
Another fundamental aspect for transfection grade DNA is the purity. In addition to being free of any nucleic acid and chemical contaminants, these DNA samples must also be entirely endotoxin free. Endotoxin is a lipopolysaccharide of the cell wall of Gram negative bacteria like E. coli that affects tissue culture cell line viability, reduces transfection efficiency, and ultimately affects the quality and outcomes of experiments. For these reasons commercially available kits provide extra washing steps to favor the removal of this agent to increase transfection success.
In conclusion, it is important for you to establish in advance how you want to utilize your plasmid DNA, so that you can decide what will be the optimal type of plasmid preparation in terms of quantity and purity.
Additional resource on the Addgene blog
- Browse blog posts on plasmid cloning
- Read our Plasmids 101 blog posts
- Learn about the differences between transformation, transduction, conjugation, and transfection
Resources on Addgene.org
- Read the molecular biology reference
- Find plasmids for your research
- Brush up on molecular biology protocols using our video collection
Topics: Plasmid Cloning, Plasmids







Leave a Comment