Before you reach for that silica spin column, stop to consider some ways to purify DNA without a kit. DNA purification kits have advantages: they are convenient and provide uniform, consistent results. But they are also less accessible due to their expense and requirement for lab equipment. Plus they create plastic waste. Kits can also have the annoying tendency to runout right when you need them and to accumulate a bunch of unused buffers because you’ve run out of columns.
Kit-less DNA purification methods avoid many of the drawbacks of kits and typically follow the same principles: cells are lysed, RNAs and proteins are removed to leave you with DNA. This DNA is then washed, and either eluted from a fixed surface or precipitated, dried, and then rehydrated. There are some slight modifications depending on the type of DNA you’re purifying (plasmid, genomic, or DNA fragments from agarose).
In this article, we will go through the basics of four methods for DNA purification without a kit as well as one way to reuse silica columns from DNA purification kits.
Kit-free alkaline lysis plasmid miniprep
Starting material: 2 mL bacterial culture
Product: plasmid DNA
This kit-free plasmid miniprep protocol from Addgene follows a similar workflow as a column-based plasmid extraction kit. First, you lyse the bacteria and denature the DNA and proteins in solution. The pH is then lowered using a renaturing solution, which causes the proteins and genomic DNA to precipitate. Plasmid DNA is free in solution. Proteins and genomic DNA are removed by centrifugation. At this point, the plasmid DNA sample can be treated with RNase to remove RNA contamination and you can use a phenol chloroform extraction to remove remaining proteins and RNase. Finally, DNA is precipitated with alcohol and resuspended in TE buffer. After this, the DNA is ready for use!
Plasmid purification and DNA gel extraction with glass syringe filters
Starting material: bacterial cultures or agarose gel slices
Product: plasmids or DNA fragments
The plasmid purification and DNA gel extraction method described in Kim and Morrison is nearly identical to that of commercially available kits but instead of binding DNA to silica columns, it’s bound to glass syringe filters. Normally DNA does not bind silica or glass, but the addition of a high concentration of a chaotropic salt (guanidine hydrochloride for the plasmid purification protocol and guanidine isothiocyanate for the gel extraction protocol) disrupts the DNA’s hydrogen bonds with water molecules and coaxes it into sticking to the hydrophobic glass filter. Each glass filter can capture up to 150 ug of DNA, and filters can be stacked together in series to increase binding capacity.
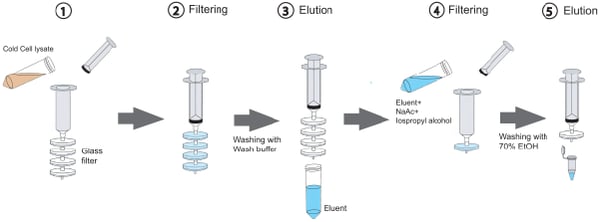 |
| Figure 1: Purify DNA using glass filters. Image: Kim and Morrison, 2009. |
This method provides DNA yield and quality similar to that obtained with commercial kits, but in less time. The authors estimate that a gravity-based column plasmid maxiprep kit takes 1-1.5 hours to complete, while this syringe-based method can be completed in 20 to 30 minutes. Additionally, while commercial plasmid prep kits come in discreet sizes (mini, midi, maxi, giga) which requires you to buy different kits for different purposes, with the Kim and Morrison method, you can just adjust the number of glass filters you use depending on the size of bacterial culture you’re working with. Overall, the glass syringe filter method is not only cheaper, but also faster and more flexible than commercial kits.
DNA gel extraction with glass milk
Starting material: agarose gel slices
Product: DNA fragments
You might be wondering, “How is milk going to help me extract DNA from agarose gel?!”
The DNA gel extraction with glass milk method from the Bishop Lab at the University of Chicago doesn’t use actual milk to purify DNA, but rather a glass powder slurry that has a milk-like appearance. To make DNA stick to the glass particles in glass milk, gel slices are first placed in a chaotropic salt sodium iodine (NaI) solution. NaI has two purposes in this protocol: 1) it solubilizes both DNA and agarose, and 2) it helps DNA stick to the glass. After binding DNA, the glass particles are pelleted by centrifugation and washed before DNA is eluted with water or TE.
The Bishop Lab’s glass milk protocol is adapted from the 1979 Patterson Protocol. When published, the Patterson Protocol was shown to purify DNA ranging in size from 100 bp to 48 kb with high yields and without degradation during the extraction procedure. Additionally, purified DNA was free of agarose and could be digested with restriction enzymes.
Cellulose nucleic acid purification
Starting material: plant, microbe, blood, or mammalian cells or tissue
Product: total nucleic acids
While glass or silica are commonly used to bind and purify DNA, cellulose also efficiently binds, or at least entraps nucleic acids. The Botella lab took advantage of this property and developed a protocol that uses Whatman No. 1 paper or even paper towels to purify DNA and RNA from plants, microbes, blood or mammalian cells in 30 seconds or less without using a pipette.
Here’s how this method works: nucleic acids are broken out of cells with a solution of salts, detergent, and ball bearings. Then a cellulose dipstick is dipped into this mixture to absorb the nucleic acids. Containinants bind cellulose less tightly than nucleic acids, so placing the dipstick into a wash solution removes contaminants while nucleic acids stick around. Finally, purified total nucleic acids are released by dipping the cellulose directly into a PCR buffer, where DNA or RNA can be amplified. Compared to a commercial magnetic bead-based kit, this cellulose-based method was faster, cheaper, and had a similar level of sensitivity.
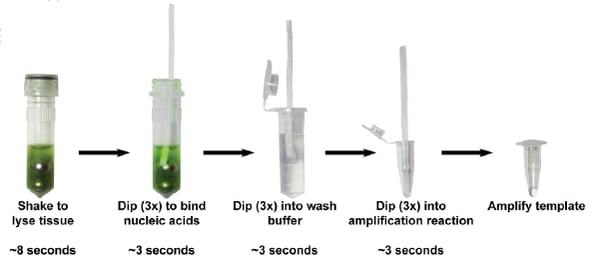 |
| Figure 2: Steps to purify DNA using a cellulose dipstick. Image: Zou et al., 2018. |
Regeneration of silica columns for DNA purification
Starting material: PCR product or agarose gel slices
Product: purified PCR product or DNA fragments
Maybe you’re not ready to give up DNA purification kits, but you’d like to reduce your lab’s waste footprint. The Zhao and Deng labs developed a simple method for regenerating silica columns that come with PCR product purification or DNA gel extraction kits. This method takes less than 30 minutes and only requires incubating the columns with 1M phosphoric acid for 3 minutes followed by a short centrifugation for a total of four times before a wash with ddH2O and then ethanol. After regeneration, trace amounts of DNA (3 femtograms/ul) could still be eluted from the column, as detected by qPCR, but this did not reduce cloning efficiencies.
When compared to fresh columns, regenerated columns had similar DNA yields. Additionally, columns regenerated up to five times had similar DNA yields as fresh columns, suggesting that columns can be reused at least five times. DNA purification kits don’t come with enough buffer for this many uses of the columns, but the Zhao and Deng labs found they could just make their own buffers when the kits buffers ran out. Homemade buffers performed the same as those provided with the kit. Although not tested by the authors, it’s possible that used plasmid purification columns could also be cleaned with 1M phosphoric acid and re-used, but trace contamination could interfere with interpreting the results of transfection assays.
Go forth and perform your DNA extractions without kits!
Regardless of the type of DNA you want to purify or your motivation of wanting to go kit-less, there’s a method for you! And even if you’re not ready to break-up with your DNA purification kit, you can still reduce the number of spin columns you need to complete your research. Do you know of other nucleic acid purification methods not mentioned in today’s post? Let us know in the comments!
Additional resources on the Addgene blog
- Read our Plasmids 101 blog series
- Find other plasmid protocols and tips
- Browse our plasmid cloning blog posts
Resources on Addgene.org
- Find protocols for molecular biology and cloning
- Read our Molecular Biology Reference
- Visit our protocol video library
References
Kim, Y., & Morrison, S.L. (2009). A Rapid and Economic In-House DNA Purification Method Using Glass Syringe Filters. PloS one. https://doi.org/10.1371/journal.pone.0007750
Vogelstein, B., & Gillespie, D.A. (1979). Preparative and analytical purification of DNA from agarose. Proceedings of the National Academy of Sciences of the United States of America, 76 2, 615-9 . https://doi.org/10.1073/pnas.76.2.615
Zhou, Y., Zhang, Y., He, W., Wang, J., Peng, F., Huang, L., Zhao, S., & Deng, W. (2018). Rapid Regeneration and Reuse of Silica Columns from PCR Purification and Gel Extraction Kits. Scientific Reports. https://doi.org/10.1038/s41598-018-30316-w
Zou, Y., Mason, M.G., Wang, Y., Wee, E.J., Turni, C., Blackall, P.J., Trau, M., & Botella, J.R. (2017). Nucleic acid purification from plants, animals and microbes in under 30 seconds. PLoS biology. https://doi.org/10.1371/journal.pbio.2003916






Leave a Comment