This post was contributed by Kusumika (Kushi) Mukherjee, editor of Trends in Pharmacological Sciences, a Cell Press reviews journal.
Stem cells are special types of cells that can develop or “differentiate” into more specialized cells with specific functions [1]. In many tissues, stem cells serve to replenish/replace damaged cells that no longer function adequately [1]. Stem cells’ ability to differentiate into multiple cell types makes them useful models for developmental processes and promising therapeutic tools. The two unique characteristics that define stem cells are:
- Their ability to self-renew or replicate while maintaining the undifferentiated “stem” state and,
- Their potency or capability to differentiate into specialized cell types [2].
We general speak of 4 levels of stem cell potency:
- Totipotency: When a cell has the ability to produce all of the differentiated cells in an organism, including the embryonic and extraembryonic cell types. In mammalian development, cells found in the zygote through to the morula stage are totipotent [2-4].
- Pluripotency: When a cell has the ability to differentiate into cells of any of the three germ layers: endoderm, ectoderm, and mesoderm. In essence, pluripotent cells can be thought of as descendants of totipotent cells. An example of pluripotent cells is embryonic stem cells (ESCs), which I discuss in detail below [2].
- Multipotency: When a cell can differentiate into multiple cell types of the same lineage. Blood (hematopoietic) stem cells are a good example of multipotent cells.
- Unipotency: As the name suggests, cells under this category can differentiate into only one cell type. Muscle satellite cells found in the adult muscle are unipotent. They can differentiate only into myogenic precursor cells [5].
Classification of stem cells
Stem cells are classified into two broad types according to their source:
- Embryonic stem cells
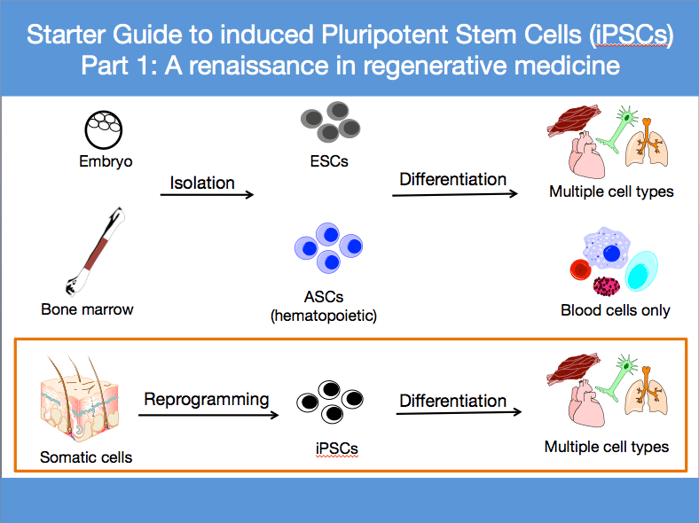
ESCs are pluripotent stem cells that are able to form all adult cell types and are obtained from the inner cell mass (ICM) of a 5-6 day old human blastocyst [6]. They are isolated mostly from abnormal and/or supernumerary preimplantation embryos that are produced during in vitro fertilization treatments and donated afterwards for ESC research purposes, with the informed consent of the donor [7].
- Adult stem cells (ASCs)
Also known as somatic stem cells, these multipotent stem cells are located in small quantities in specific “stem cell niches” found in many organs in the adult human body. These generate specialized cells for their location, mostly to maintain cell numbers or replenish dying cells. For example, hematopoietic stem cells found in the bone marrow only differentiate into cells of peripheral blood. Typically, there is a very small number of ASCs in each tissue and once removed from the body; their ability to self-renew is greatly diminished.
Embryonic and adult stem cells can be used for different research and therapeutic purposes. Both types have their advantages and disadvantages. Here is a table listing some of them:
| Characteristic | ESC | ASC |
| Self-renewal | High | Low once isolated from the human body |
| Usage | Universal. Can generate all adult cell types. | Limited. Only specific cell types can be produced |
| Ethical concern | High. Embryos are destroyed during ESC isolation. | Low |
| Chances of post-transplantation rejection when used therapeutically | High | Low (if ASCs are isolated from the patient) |
For many years, ESCs or ASCs were the only realistic options for scientists working in regenerative medicine, but the longing for a model system which combined the advantages of both while limiting disadvantages led to the creation of induced pluripotent stem cells or iPSCs.
Induced pluripotent stem cells
iPSCs are stem cells that are obtained by reprogramming differentiated adult cells by the ectopic expression of four embryonic genes- OCT4, SOX2, KLF4, and C-MYC. iPSCs were first generated from mouse fibroblasts in 2006 in Shinya Yamanaka’s lab, where the reprogramming factors were delivered via retroviral infection [8]. The work was taken forward the next year when both the Yamanaka and Thomson labs showed the successful generation of human iPSCs from dermal fibroblasts via retroviral and lentiviral infections respectively [9, 10]. These stellar works led to a Nobel Prize for Dr. Yamanaka in 2012.
Find plasmids for generating iPSCs
iPSCs and ESCs closely resemble each other in many aspects. Like ESCs, iPSCs have a high self-renewal rate and can be used universally to generate many adult cell types. Like ESCs, iPSCs can differentiate in vitro into derivatives of all three germ layers, form embryoid bodies, and form teratomas [11]. iPSCs also possess similar morphologies, growth properties, and express stem cell markers similar to ESCs [11].
While iPSCs and ESCs have similar functional utility, iPSCs bypass the transplantation compatibility issues and ethical concerns of ESCs. As iPSCs can be sourced directly from a patient’s adult cells when used therapeutically, the chances of post-transplantation rejection are low. Further, as iPSC generation does not require embryos, their use skirts issues surrounding the use of embryos in research. With all these benefits, iPSCs have increasingly become the ideal experimental system for research in regenerative medicine over the last decade.
In the next post of this series, we’ll discuss the two widely used approaches of somatic cell conversion: conversion via iPSC formation, commonly referred to as reprogramming and, direct conversion from one somatic cell type to another, also known as transdifferentiation.
Many thanks to our guest blogger, Kusumika (Kushi) Mukherjee.
 Kusumika (Kushi) Mukherjee is the Editor of Trends in Pharmacological Sciences, a Cell Press reviews journal. She joined Cell Press to pursue a career in science communication and publishing after completing her postdoctoral training from Massachusetts General Hospital and Harvard Medical School. Connect with her on LinkedIn @ https://www.linkedin.com/in/
Kusumika (Kushi) Mukherjee is the Editor of Trends in Pharmacological Sciences, a Cell Press reviews journal. She joined Cell Press to pursue a career in science communication and publishing after completing her postdoctoral training from Massachusetts General Hospital and Harvard Medical School. Connect with her on LinkedIn @ https://www.linkedin.com/in/
References
1. Dittrich, R., M.W. Beckmann, and W. Wurfel, Non-embryo-destructive Extraction of Pluripotent Embryonic Stem Cells: Implications for Regenerative Medicine and Reproductive Medicine. Geburtshilfe Frauenheilkd, 2015. 75(12): p. 1239-1242. PubMed PMID: 26726264. PubMed Central PMCID: PMC4686367.
2. Jaenisch, R. and R. Young, Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell, 2008. 132(4): p. 567-82. PubMed PMID: 18295576. PubMed Central PMCID: PMC4142810.
3. Mitalipov, S. and D. Wolf, Totipotency, pluripotency and nuclear reprogramming. Adv Biochem Eng Biotechnol, 2009. 114: p. 185-99. PubMed PMID: 19343304. PubMed Central PMCID: PMC2752493.
4. Bongso, A. and M. Richards, History and perspective of stem cell research. Best Pract Res Clin Obstet Gynaecol, 2004. 18(6): p. 827-42. PubMed PMID: 15582541.
5. Seale, P., A. Asakura, and M.A. Rudnicki, The potential of muscle stem cells. Dev Cell, 2001. 1(3): p. 333-42. PubMed PMID: 11702945.
6. Cowan, C.A., et al., Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med, 2004. 350(13): p. 1353-6. PubMed PMID: 14999088.
7. Turetsky, T., et al., Laser-assisted derivation of human embryonic stem cell lines from IVF embryos after preimplantation genetic diagnosis. Hum Reprod, 2008. 23(1): p. 46-53. PubMed PMID: 17989069.
8. Takahashi, K. and S. Yamanaka, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 2006. 126(4): p. 663-76. PubMed PMID: 16904174.
9. Takahashi, K., et al., Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 2007. 131(5): p. 861-72. PubMed PMID: 18035408.
10. Yu, J., et al., Induced pluripotent stem cell lines derived from human somatic cells. Science, 2007. 318(5858): p. 1917-20. PubMed PMID: 18029452.
11. Medvedev, S.P., A.I. Shevchenko, and S.M. Zakian, Induced Pluripotent Stem Cells: Problems and Advantages when Applying them in Regenerative Medicine. Acta Naturae, 2010. 2(2): p. 18-28. PubMed PMID: 22649638. PubMed Central PMCID: PMC3347549.
Additional Resources on the Addgene Blog
- pCXLE Toolkit
- Stem Cell Models for Disease and Open Science: An Interview with Darrell Kotton
- Four Factors that Differentiate the Stem Cell Field
Resources on Addgene.org
- Visit Our Stem Cell Page
- Find Plasmids for Reprogramming
- Find Plasmids for Differentiation
Topics: Stem Cells, Other





Leave a Comment