This post was contributed by Samuel Mortensen, a PhD candidate at Northeastern University.
Working with plants doesn’t always have to be a time-consuming process. While developing transgenic hairy root lines in tissue cultures takes half a year, and generating a transgenic plant can take even longer, a transient plant leaf transformation process could save the plant biologist some time… months, in fact.
 In the Lee-Parsons lab, we are investigating how the production of cancer therapeutic drugs, vinblastine and vincristine, are regulated in the medicinal plant, the Catharanthus roseus (Figure 1). The ultimate goal of our research is to enhance their production either in the plant or in plant tissue cultures.
In the Lee-Parsons lab, we are investigating how the production of cancer therapeutic drugs, vinblastine and vincristine, are regulated in the medicinal plant, the Catharanthus roseus (Figure 1). The ultimate goal of our research is to enhance their production either in the plant or in plant tissue cultures.
C. roseus is not a model organism such as Arabidopsis or tobacco. Studying gene function in C. roseus is strongly limited by the availability of methods. Infection with Agrobacterium rhizogenes can be used to develop stable hairy root lines in tissue culture. This method is commonly used in C. roseus, but processes specific to photosynthetic active tissues cannot be studied in hairy roots, as functional chloroplasts and leaf specific cell types are missing in roots. The development of transgenic hairy roots takes at least six months. Generating a transgenic plant takes even longer, is more labor intensive, inefficient, and often not reproducible. Prior to creating transgenic cultures or plants, our transient expression method enables us to quickly screen and investigate transcription factor candidates and the regulation of genes in the biosynthetic pathway.
Why plant scientists do transient leaf transformations
The development of transgenic plants is a common problem in non-model organisms. Therefore, transient methods are usually the first choice for investigating gene function, as these experiments can be done in days to weeks instead of months to years. In the plant sciences “transient transformation” usual means that neither a stable cell line or plant is regenerated from the transformed tissue. Gene function is commonly studied by transiently silencing and/or overexpressing a gene of interest in the plant.
A common method for silencing genes
Virus induced gene silencing (VIGS) is a transient technique that uses a virus and the plant's post-transcriptional machinery to silence genes of interest (Unver and Budak, 2009). This method works well in C. roseus.
A common method for overexpressing genes
In plants like Arabidopsis and tobacco, Agrobacterium infiltration into leaves is a simple and efficient way to overexpress a gene and evaluate its function. In nature Agrobacterium contains a plasmid with a T-DNA (transfer-DNA) region. During infection this T-DNA region is transferred into the plant. In the laboratory we can utilize this natural process by replacing the genes on the T-DNA region with our gene of interest or a reporter gene so that the transformation and gene expression can be tracked. During a transient leaf transformation, the engineered Agrobacterium strain is brought into contact with the leaf tissue and the T-DNA region with the gene of interest is transferred into the cells. But for C. roseus, Agrobacterium infiltration into leaves does not work well, as the leaves have a waxy texture and are difficult to infiltrate.
We have recently overcome this problem in C. roseus by working with young seedlings and optimizing several other aspects of the transient transformation procedure (Mortensen et al., 2019). Our method might provide some useful guidelines for the development of transient transformation systems for other species as well.
Things to consider in developing a transient leaf transformation method with Agrobacterium:
If you are new to the field, the following are useful tips for your first transient leaf transformation:
Check if there are working protocols available for your species
This will save you a lot of time. Don't re-invent the wheel.
Test different leaf ages
Usually younger leaves work better. Not every leaf is equally susceptible to transient transformation.
Choose good reporter genes
There are many great reporter genes available and which one you choose depends on your experiment (de Ruijter et al., 2003). For example, we did our initial transformations with the GUS gene to see if the transformation was successful at all. Once we were on the right track, we switched to luciferases to more easily quantify the effect of the different treatments on transformation success. Some reporter gene examples are below.
- The GUS gene (β-glucuronidase) with histochemical GUS-staining is a very useful visual reporter (Figure 2). The GUS enzyme cleaves a substrate, which will then form a blue precipitate. GUS is usually the first choice for establishing a new transformation protocol as it can easily show which tissue have been transformed. But caution: check for GUS-like activity of your tissue. Run a control with Agrobacterium lacking the GUS gene since plants can have endogenous GUS-like activity (Hu et al., 1990). We commonly use pSB161 - pL2_pSB90_2x35S::GUS::tMAS to confirm transformation success.
- Luciferases are great quantitative reporters and use a substrate to produce bioluminescence. Luciferases usually have shorter mRNA and protein half-lives then GUS and are therefore good temporal reporters to measure promoter activity, for example. Useful positive controls could include: pSB96 - pL2_pSB90_2x35S::fLUC-I::tNOS & pSB166 - pL2_pSB90_tMAS::rLUC-I::pMAS.
- Fluorescent proteins (FP, e.g. GFP) can be used in many ways. Often a FP is fused to a protein of interest to elucidate the subcellular localization of your protein of interest.
Find all plasmids from the Lee-Parsons lab.
Test different leaf infiltration methods
- Co-cultivation (Li et al., 2009): The co-cultivation of Agrobacterium with your leaves is a very easy transformation method, but can be inefficient in many species. More efficient are syringe or vacuum infiltration.
- Syringe infiltration (D'Aoust et al., 2009): Usually a syringe without a needle is used to inject an Agrobacterium culture through the stomata into the abaxial site (lower surface) of the leaf. This is a simple and easy technique to implement. Success might vary between experimenters.
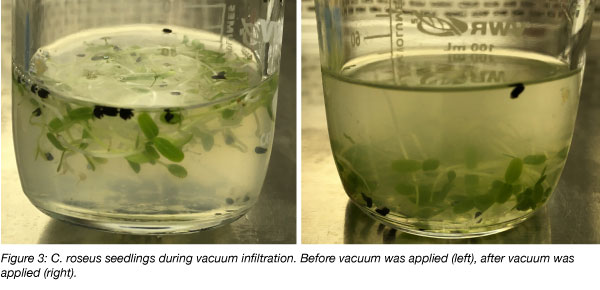
- Vacuum infiltration (Mortensen et al., 2019): Leaves or whole plants are placed in an Agrobacterium solution and a vacuum is applied with a vacuum bell or desiccator. Successful infiltration can often be seen by sinking of the tissue (see Figure 3). This method is probably the most scalable method.
Transfer your plant into the dark 16 hours before the transformation.
We routinely transfer our plants 16 hours before the transformation into the dark and leave them an additional two days in the dark after the transformation.
Use introns
Plant promoters can be recognized or leaky in Agrobacterium. This is especially problematic with reporter genes as you might observe expression from Agrobacterium, but not the plant. For example, we have seen strong expression of the GUS gene with the commonly used CaMV 35S promoter in Agrobacterium. This can be easily avoided by the use of introns, which will be removed from the transcripts in the plant but not from Agrobacterium.
Test different Agrobacterium strains
We used the disarmed Agrobacterium tumefaciens strain GV3101(pMP90), which works well for us.
Use a well working vector system
This allows you to quickly assemble vectors and test different ideas. We are a huge fan of the Golden Gate based MoClo system. The vectors mentioned in this blog post above are ready to use vectors, but with the MoClo system you can easily and quickly create customized vectors. For more information on MoClo for plants, check out this interview with the Nicola Patron, who shared the MoClo plant kit via Addgene.
For us, transient leaf transformations are a great help in screening candidate genes and speeding up research. Certainly, there is still more to transient leaf transformations, but we hope that these tips might help to get you started. Once you have established a well working protocol in your lab, it can become a strong tool for your research. Feel free to add further tips and experiences in the comments below.
Many thanks to our guest blogger Samuel Mortensen from Northeastern University.
 Samuel Mortensen is a PhD candidate at Northeastern University in Boston, MA. He obtained his BSc and MSc in Plant Biotechnology from Leibniz Universität Hannover (Germany), before starting his work in the Lee-Parsons Lab.
Samuel Mortensen is a PhD candidate at Northeastern University in Boston, MA. He obtained his BSc and MSc in Plant Biotechnology from Leibniz Universität Hannover (Germany), before starting his work in the Lee-Parsons Lab.
References
D'Aoust, Marc-André, et al. "Transient expression of antibodies in plants using syringe agroinfiltration." Recombinant proteins from plants. Humana Press, 2009. 41-50. PubMed PMID: 19183892.
De Ruijter, N. C. A., et al. "Evaluation and comparison of the GUS, LUC and GFP reporter system for gene expression studies in plants." Plant Biology 5.02 (2003): 103-115.
Hu, Ching-yeh, et al. "Intrinsic GUS-like activities in seed plants." Plant cell reports 9.1 (1990): 1-5. PubMed PMID: 24226366.
Lee, Min Woo, and Yinong Yang. "Transient expression assay by agroinfiltration of leaves." Arabidopsis Protocols. Humana Press, 2006. 225-229. PubMed PMID: 16739580.
Li, Jian-Feng, et al. "The FAST technique: a simplified Agrobacterium-based transformation method for transient gene expression analysis in seedlings of Arabidopsis and other plant species." Plant methods 5.1 (2009): 6. PubMed PMID: 19457242. PubMed Central PMCID: PMC2693113.
Mortensen, Samuel, et al. "EASI transformation: An efficient transient expression method for analyzing gene function in Catharanthus roseus seedlings." Frontiers in plant science 10 (2019). PubMed PMID: 31263474. PubMed Central PMCID: PMC6585625.
Unver, Turgay, and Hikmet Budak. "Virus-induced gene silencing, a post transcriptional gene silencing method." International journal of plant genomics 2009 (2009). PubMed PMID: 19547658. PubMed Central PMCID: PMC2699436.
Additional resources on the Addgene blog
- Read all of our plant biology blog posts
- Find tips for Arabidopsis transformation
- Read our Plasmids 101 blog posts
Resources on Addgene.org
- Browse our Plant Plasmids and Resources page
- Find synthetic biology plant plasmids
- Find CRISPR plant plasmids
Topics: Plant Biology, Molecular Biology Protocols and Tips, Other







Leave a Comment