Integrins are essential cell surface receptors involved in complex signaling pathways and the linkage of intra- and extracellular environments. Found on nearly every cell in the human body, integrins are key to a diverse array of processes, including cell adhesion, migration, and signaling, as well as immune response, tissue repair, and kidney development. Despite their ubiquity and significance, these receptors have proved challenging to study. Cells often express multiple integrins that bind the same ligand, making it difficult to dissect the function of one integrin unless others are inhibited.
Recently, we at the Institute for Protein Innovation (IPI), a Boston-based, nonprofit protein research institute, partnered with Addgene to make our collection of integrin antibodies available through Addgene’s repository. The collection includes 26 unique antibodies that bind discrete subunits or particular subunit pairs. Some of the antibodies in the collection are able to block ligand binding — and therefore interrupt integrin function — providing researchers new tools to identify integrin presence, study signaling mechanisms, or ascertain which integrins are responsible for specific ligand-binding functions, all key to basic biomedical research and drug development.
Integrin structure and conformational complexity
There are 24 known mammalian integrins, all heterodimers composed of one of 18 alpha and one of eight beta subunits. Both subunits have short intracellular ends and large ectodomains containing ligand-binding heads and long legs that protrude from the cell membrane, physically connecting the extracellular environment and the cytoskeleton.
Most integrins have three conformational states: bent-closed, extended-closed, and extended-open. In the two closed states, integrins are inactive, with low affinity for ligands. When integrins interact with extracellular ligands, their ectodomains can extend and open, binding ligands with high affinity.
This extended-open state is key to strengthening ligand binding, mediating cell adhesion and migration, initiating intracellular signal cascades, and allowing agile responses to mechanical forces in the cell’s microenvironment. The actin cytoskeleton exerts tensile force, transmitted through the integrin, where it is resisted by the ligand embedded in the extracellular environment. This further stabilizes the extended open integrin conformation. During embryonic development, extended-open integrins enable tissue morphogenesis, organ development, and the shaping of the extracellular matrix. During immune responses, this same conformation encourages immune cell adhesion and migration into inflamed tissues.
Integrins' conformational flexibility is crucial for their function. With the right research reagents, researchers can measure this conformational change to investigate the role of specific integrins in cellular processes.
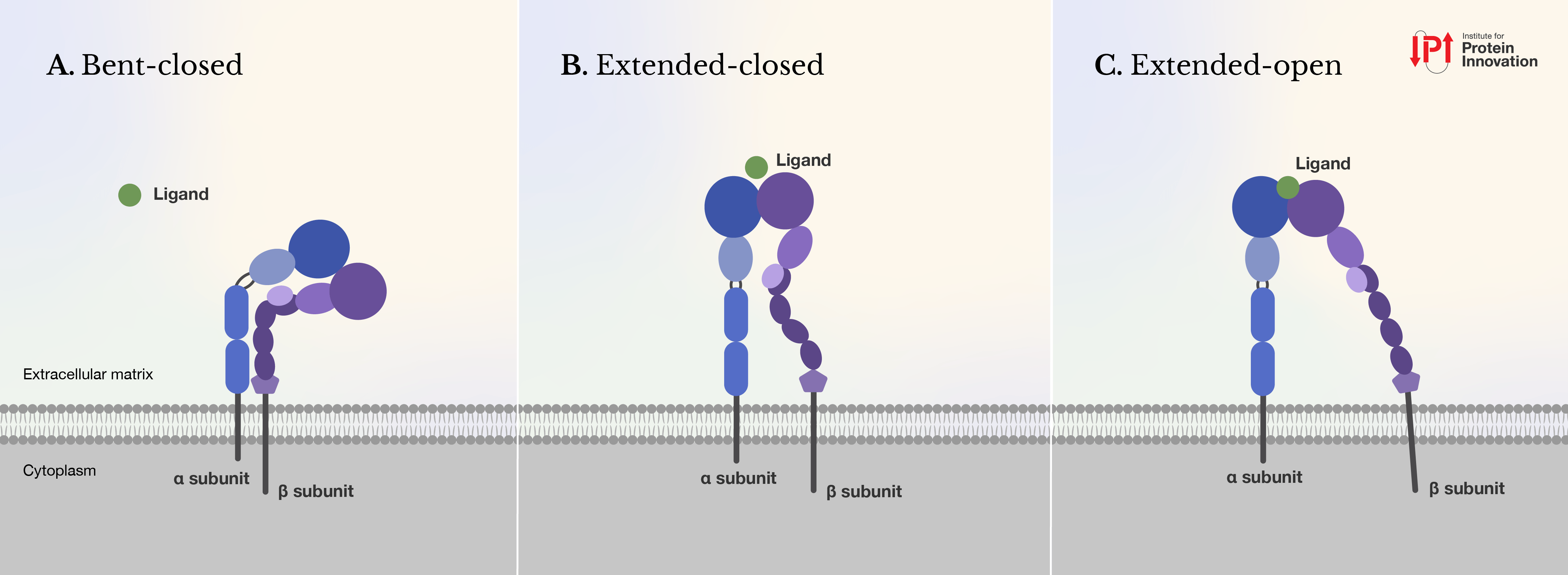 |
| Figure 1: Integrins can shape into three conformations: (A) bent-closed, (B) extended-closed, and (C) extended-open. When a ligand binds, it typically shifts the integrin from its closed to its open state, activating the receptor. Ligands can bind to integrins in their closed states (A) and (B) but with much lower affinity than their open states (C). Figure courtesy of IPI. |
IPI anti-integrin toolset
IPI’s anti-integrin toolset comprises 26 recombinant antibodies (rAbs) either produced de novo using yeast display technology or produced from hybridoma antibodies developed in the Springer lab. Some are rabbit immunoglobulin G1 (IgG1) chimeric antibodies, and others are rat or mouse IgG2a. Additional human IgG1s are also available on demand.
The 11 IPI synthetic rAbs, developed using yeast display technology, are directed to integrins that bind the Arginine-Glycine-Aspartate (RGD) amino acid sequence in their ligands. This well-known motif serves as a recognition site for one subfamily of integrins, the RGD-binding integrins. Six of the 11 IPI antibodies contain RGD-mimetic sequences that imitate this motif and compete with both small molecule integrin inhibitors and natural ligands, such as fibronectin, to block receptor function, e.g., cell adhesion. This pocket-binding property in an antibody is extremely rare. While many function-blocking integrin antibodies exist, almost all bind outside the ligand-binding pocket, their sheer size impeding ligand attachment. The five other IPI rAbs bind RGD-binding integrins at non-ligand-binding sites and do not interfere with integrin function.
Overall, the synthetic rAbs demonstrate remarkable specificity for their targeted integrin subtype, even when in competition with other similar integrins, as reported by IPI and the Springer lab (Hao et al., 2024). In this study, we, along with the Springer lab, reported the binding specificity, kinetics, and affinity of these 11 newly discovered synthetic rAbs. We then used these antibodies to show the binding preferences of the αV subunit and its β partners. Of interest, we demonstrated that anti-integrin αVβ5.9 “essentially completely blocks all adhesion,” inhibiting the function of αVβ5; to our knowledge, this is the first such antibody to be developed and defined. According to the Springer lab, a highly specific function-blocking antibody, αVβ3.13, may also block function of mouse αVβ3, again the first known reagent to do so.
The 15 hybridoma-derived antibodies fall into two groups. Seven are reconstructions of the original hybridomas as new reproducible rat or mouse rAbs. The other eight are chimeric antibodies composed of rat or mouse variable regions fused with rabbit constant domains. All bind specific integrin alpha subunits, including αL, αM and α5.
IPI’s anti-integrin rAbs are unconjugated and recommended for use in flow cytometry. Based on the established applications of the original hybridoma monoclonals, we anticipate that our chemically identical hybridoma-derived antibodies will also work in immunoprecipitation and immunohistochemistry.
Table 1: IPI antibodies
|
Antibody |
Target Proteins | Reactivity | Isotype | Source Species |
| Integrin alpha-5,Integrin beta-1 | Human, Mouse | IgG1 | Rabbit | |
| Integrin alpha-5,Integrin beta-1 | Human, Mouse | IgG1 | Rabbit | |
| Integrin alpha-V,Integrin beta-8 | Human | IgG1 | Rabbit | |
| Integrin alpha-V,Integrin beta-8 | Human | IgG1 | Rabbit | |
| Integrin alpha-V,Integrin beta-6 | Human, Mouse | IgG1 | Rabbit | |
| Integrin alpha-V,Integrin beta-6 | Human, Mouse | IgG1 | Rabbit | |
| Integrin alpha-V,Integrin beta-6 | Human, Mouse | IgG1 | Rabbit | |
| Integrin alpha-V,Integrin beta-6 | Human, Mouse | IgG1 | Rabbit | |
| Integrin alpha-V,Integrin beta-5 | Human, Mouse | IgG1 | Rabbit | |
| Integrin alpha-V,Integrin beta-3 | Human, Mouse | IgG1 | Rabbit | |
| Integrin alpha-V,Integrin beta-3 | Human, Mouse | IgG1 | Rabbit | |
| Integrin alpha-L | Human | IgG1 | Rabbit | |
| Integrin alpha-L | Mouse | IgG1 | Rabbit | |
| Integrin alpha-L | Human | IgG1 | Rabbit | |
| Integrin alpha-M | Mouse, Human | IgG1 | Rabbit | |
| Integrin alpha-M | Human | IgG1 | Rabbit | |
| Integrin alpha-M | Human | IgG1 | Rabbit | |
| Integrin alpha-M | Human | IgG1 | Rabbit | |
| Integrin alpha-5 | Mouse | IgG1 | Rabbit | |
| Integrin alpha-L | Human | IgG2a | Mouse | |
| Integrin alpha-L | Mouse | IgG2a | Rat | |
| Integrin alpha-M | Mouse, Human | IgG2a | Rat | |
| Integrin alpha-M | Human | IgG2a | Mouse | |
| Integrin alpha-M | Human | IgG2a | Mouse | |
| Integrin alpha-M | Human | IgG2a | Mouse | |
| Integrin alpha-5 | Mouse | IgG2a | Rat |
Targeting integrins in the lab
The Ha and Springer labs recently showed RGD-binding integrins demanded higher tensions before they would enable cells to spread, resulting in cells that elongated and morphed asymmetrically (Jo et al., 2022). By contrast, LVDP-binding integrin α4β1 required and conferred less force, leading cells to spread into symmetrical circles. While elucidating the role of tension, however, the researchers realized they needed specific tools to interpret the roles of individual integrins. The Ha lab — in collaboration with the Springer lab, the Jaumouillé lab, the Waterman lab, and IPI — used IPI antibodies and affinity reagents to study the difference in cell morphology changes induced by integrins binding the RGD motif compared to those binding the Leucine-Valine-Aspartate-Proline (LVDP) motif.
The Ha and Springer teams employed IPI synthetic antigen-binding fragments (Fabs) that bound an amino acid region displayed by a subset of the RGD-binding integrins containing the αV subunit. Using these affinity reagents, the investigators inhibited other RGD-binding integrins, individually and in combination, showing that among the three αV integrins expressed on the BJ-5ta fibroblast cells, integrin αVβ1 was critical in initiating cell spreading and two other RGD-binding integrins, αVβ3 and αVβ5, participated but were not required for cell adhesion. The results surprised the investigators, challenging the long-held belief that αVβ3 would be the most functionally important integrin on these cells.
What’s next
IPI is working with Addgene to accelerate biomedical research by distributing antibodies developed by IPI. Our integrin antibodies are the first deposit from an expanding collection of readily accessible recombinant antibodies and plasmids.
To encourage collaboration and education in protein science, IPI hosts events and training courses on technologies and laboratory techniques related to antibody discovery. You can find cell surface receptor biology talks on the IPI website and stay informed on future events by signing up for the IPI newsletter.
The collection of 26 integrin antibodies comes from IPI’s work with the Boston Children’s Hospital and Harvard-based lab of Tim Springer, the founder of IPI.
Caitlin Faulds is a science writer at the Institute for Protein Innovation. She writes about all things IPI, from the scientists and scientific process behind the scenes to the new antibodies and research coming out of the lab.
References and resources
References
Hao, Y., Yan, J., Fraser, C., Jiang, A., Anuganti, M., Zhang, R., Lloyd, K., Jardine, J., Coppola, J., Meijers, R., Li, J., & Springer, T. A. (2024). Synthetic integrin antibodies discovered by yeast display reveal αV subunit pairing preferences with β subunits (p. 2024.01.26.577394). bioRxiv. https://doi.org/10.1101/2024.01.26.577394
Jo, M. H., Li, J., Jaumouillé, V., Hao, Y., Coppola, J., Yan, J., Waterman, C. M., Springer, T. A., & Ha, T. (2022). Single-molecule characterization of subtype-specific β1 integrin mechanics. Nature Communications, 13(1), 7471. https://doi.org/10.1038/s41467-022-35173-w
More resources from Addgene
Topics: Antibodies






Leave a Comment