In a previous post from our Plasmids 101 series, we learned how the Cre-loxP recombination system can be used to induce site-specific recombination events, and that the orientation of the flanking loxP sites directs the Cre recombinase to invert, translocate, or excise a DNA fragment. The availability of both wild-type and mutant loxP sites has allowed scientists to leverage this system in new, creative ways. Today’s post will focus on one such strategy--the FLEx switch--which utilizes recombination elements to turn off expression of one gene, while simultaneously turning on the expression of another!
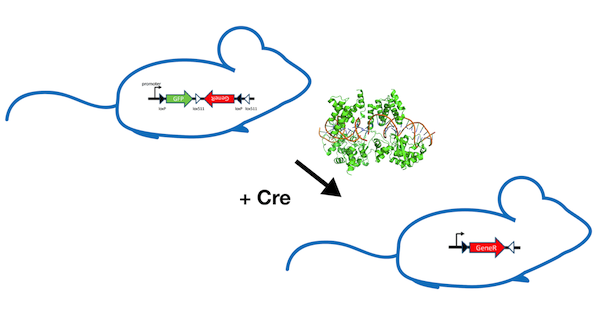
What Are FLEx switches?
FLEx (or “flip-excision”) switches were designed as a genetic tool for researchers to conditionally manipulate gene expression in vivo using site-specific recombination. The FLEx switch takes advantage of the orientation specificity of the site-specific recombinases (SSRs) Cre and FLP. SSRs bind DNA at target sites to induce site specific recombination events: Cre recombinase binds loxP sites, while FLP binds FRT sites. When a DNA sequence is flanked by target sites (floxed) in opposing orientations, a SSR will invert the DNA sequence between the sites. If a DNA sequence is floxed in the same orientation, the SSR will excise the DNA fragment. By manipulating the number, orientation, and type of target sites that flox your genes of interest, a powerful FLEx switch can be created for your specifc experimental needs.
How does the FLEx switch work?
Let’s say you want to design a genetic FLEx switch that turns BFP expression off, while turning on mCherry expression. For this FLEx switch to successfully work, the cassette would need to contain the BFP coding sequence in the sense orientation, followed by the mCherry coding sequence in the antisense orientation (Figure 1, top). The entire DNA cassette would be flanked by two pairs of target sites- one wild-type pair (loxP, black arrowheads) and one mutated pair (for example- lox511, white arrowheads). It is necessary to use two different pairs of target sites for this strategy to work effectively. Both loxP and lox511 are recognized by Cre but lox511 sites can only recombine with other lox511 sites, not with loxP sites. Alternatively, you could use a pair of loxP sites and a pair of FRT sites and include both Cre and FLP recombinases.
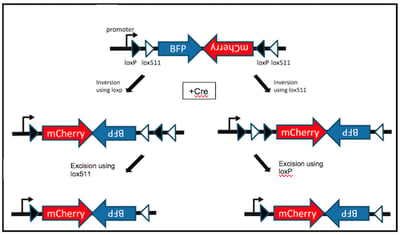 |
| Figure 1 (modified from Schnutgen et al. Nat Biotech 2003): Basic FLEx switch will express BFP in the absence of, or mCherry in the presence of Cre recombinase. |
Once the SSR of choice is introduced, recombination can proceed either by first utilizing the loxP sites or the lox551 sites. Regardless, the first recombination step will invert the intervening DNA fragment using either loxP or lox511 sites, leaving two identical sites on one end of the DNA fragment (Figure 1, middle). A second recombination event then excises the DNA between the identical loxP or lox511 sites (Figure 1, bottom). Since only one loxP and lox511 site will remain on either side of the DNA fragment, any additional recombination events are impossible even in the presence of Cre recombinase. This plasmid now specifically drives expression of mCherry instead of BFP.
Beyond switching fluorophores, why is FLEx useful?
The power of the FLEx system lies in the ability to conditionally turn off one gene and activate another. In the field of mouse genetics, FLEx is currently being used to study a wide variety of in vivo conditional gene rescue experiments, as well as for improvement of optogenetic adeno-associated virus (AAV) vectors. Listed below are a few additional uses for FLEx switches:
- Conditional rescue of a gene knockout: To test if the phenotype you are observing is really due to a single gene deleted in your knockout mouse, you can design a FLEx switch conditional knock-in cassette. As shown in Figure 2, the cassette should contain a reporter DNA sequence (such as GFP) in the sense orientation, followed by coding sequence of the gene you would like to rescue with in the antisense orientation (let’s call the gene “GeneR”). The key to this experiment is the placement of the two pairs of target sites. By flanking the reporter with one loxP site and one lox511 site in the same orientation, and placing the additional loxP and lox511 sites after the inverted GeneR sequence in the opposing orientation, you have created a cassette that will express GeneR only in the presence of Cre. In this example, Cre would first invert the reporter and GeneR fragment. Then the second recombination event would excise the reporter from the cassette, allowing for expression of GeneR. This allows a researcher to test whether or not expressing GeneR alone rescues the phenotype being studied.
- Introduction of a conditional point mutation or functional mutation in vivo: Similar to a conditional rescue experiment with a wildtype copy of a gene, you can also design FLEx switches to conditionally test the function of various point mutations and truncations in the absence of the endogenous gene.
- Spatio-temporal expression of optogenetic reporters: Since most optogenetic reporters are delivered using AAV injection, the majority of the cells surrounding the injection site will become infected and would have the potential to express the transduced reporter gene. Generating a FLEx switch to control expression of an optogenetic reporter would ensure that the reporter remains silent until a cell or tissue-specific Cre is provided. Since the FLEx switch ensures Cre-dependent reporter expression, the potential for background expression of the reporter will be greatly reduced, allowing for clearer characterization of the location, morphology, and circuit mapping of a targeted neuronal population.
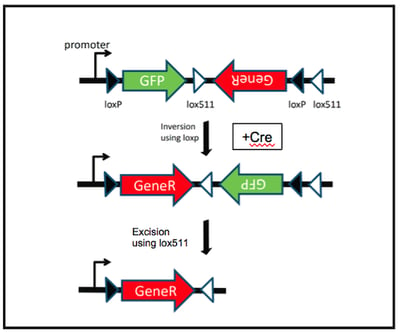 |
| Figure 2 (modified from Schnutgen et al. Nat Biotech 2003): In the presence of Cre, GFP expression is lost, allowing for rescued expression of GeneR. |
Sometimes also referred to as DiO (Double-floxed inverse Orientation), FLEx systems are generally considered "Cre-On" meaning that your gene of interest starts in the inverse/antisense "off" position and is flipped to the sense (on) orientation in the presence of Cre. A similar strategy can be employed for "Cre-off" (i.e. your gene of interest starts in the sense orientation and Cre is used to flip it to "off"). These Cre-off switches may be referred to as DO (Double-floxed Orientation) and could be used to study the physiology or behavior of certain cell populations.
Acknowledgement:
Through a partnership with genOway, we are able to distribute materials containing FLEx technology. Read the genOway press release for more information.
References:
1. Schnütgen F, Doerflinger N, Calléja C, Wendling O, Chambon P, and Ghyselinck NB. A directional strategy for monitoring Cre-mediated recombination at the cellular level in the mouse. 2003 PubMed PMID: 12665802.
2. Branda, CS and Dymecki, SM. Talking about a Revolution: The Impact of Site-Specific Recombinases on Genetic Analyses in Mice. 2004. PubMed PMID: 14723844.
3. Atasoy D, Aponte Y, Su HH, and Sternson SM. 2008. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. PubMed PMID: 18614669. PubMed Central PMCID: PMC2593125.
4. Saunders A, Johnson CA, Sabatini BL. 2012. Novel recombinant adeno-associated viruses for Cre activated and inactivated transgene expression in neurons. PubMed PMID: 2286602. PubMed Central PMCID: PMC3406316.
Find FLEx plasmids from these Addgene depositors:
Additional Resrouces on the Addgene Blog
- Read about Other Means of Genome Engineering
- Using FLEx AAV Vectors? Learn about Viral Vector Elements
- Read Our Primer on Optogenetics
Topics: Plasmids 101, Cre-lox, Plasmids







Leave a Comment